smallpox vaccine and vaccination in the intensified ... - libdoc.who.int
smallpox vaccine and vaccination in the intensified ... - libdoc.who.int
smallpox vaccine and vaccination in the intensified ... - libdoc.who.int
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
11. VACCINATION IN THE INTENSIFIED PROGRAMME<br />
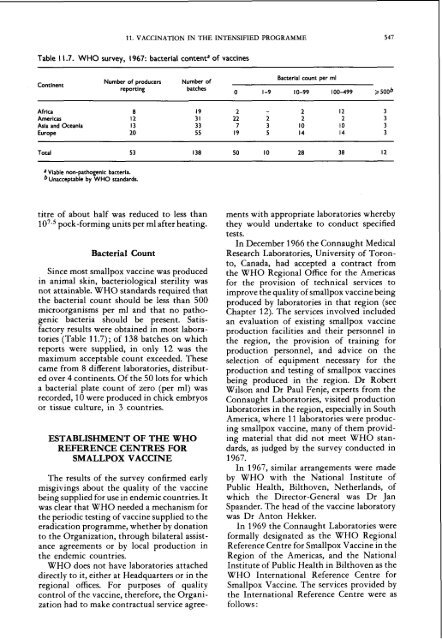
Table 1 1.7. WHO survey, 1967: bacterial contenta of <strong>vacc<strong>in</strong>e</strong>s<br />
Cont<strong>in</strong>ent<br />
Africa 8<br />
Americas 12<br />
Asla <strong>and</strong> Oceania 13<br />
Europe 20<br />
Number of producers Number of<br />
report<strong>in</strong>g batches<br />
Bacterial count per ml<br />
0 1-9 10-99 100-499 2500~<br />
Total 53 138 50 10 28 38 12<br />
a Viable non-pathogenic bacterla.<br />
bunacceptable by WHO st<strong>and</strong>ards.<br />
titre of about half was reduced to less than<br />
107.5 pock-form<strong>in</strong>g units per m1 after heat<strong>in</strong>g.<br />
Bacterial Count<br />
S<strong>in</strong>ce most <strong>smallpox</strong> <strong>vacc<strong>in</strong>e</strong> was produced<br />
<strong>in</strong> animal sk<strong>in</strong>, bacteriological sterility was<br />
not atta<strong>in</strong>able. WHO st<strong>and</strong>ards required that<br />
<strong>the</strong> bacterial count should be less than 500<br />
microorganisms per m1 <strong>and</strong> that no patho-<br />
genic bacteria should be present. Satis-<br />
factory results were obta<strong>in</strong>ed <strong>in</strong> most labora-<br />
tories (Table 11.7) ; of 138 batches on which<br />
reports were supplied, <strong>in</strong> only 12 was <strong>the</strong><br />
maximum acceptable count exceeded. These<br />
came from 8 different laboratories, distribut-<br />
ed over 4 cont<strong>in</strong>ents. Of <strong>the</strong> 50 lots for which<br />
a bacterial plate count of zero (per ml) was<br />
recorded, 10 were produced <strong>in</strong> chick embryos<br />
or tissue culture, <strong>in</strong> 3 countries.<br />
ESTABLISHMENT OF THE WHO<br />
REFERENCE CENTRES FOR<br />
SMALLPOX VACCINE<br />
The results of <strong>the</strong> survev confirmed earlv<br />
misgiv<strong>in</strong>gs about <strong>the</strong> quality of <strong>the</strong> <strong>vacc<strong>in</strong>e</strong><br />
be<strong>in</strong>g supplied for use <strong>in</strong> endemic countries. It<br />
was clear that WHO needed a mechanism for<br />
<strong>the</strong> periodic test<strong>in</strong>g of <strong>vacc<strong>in</strong>e</strong> supplied to <strong>the</strong><br />
eradication programme, whe<strong>the</strong>r by donation<br />
to <strong>the</strong> Organization, through bilateral assist-<br />
ance agreements or by local production <strong>in</strong><br />
<strong>the</strong> endemic countries.<br />
WHO does not have laboratories attached<br />
directly to it, ei<strong>the</strong>r at Headquarters or <strong>in</strong> <strong>the</strong><br />
regional offices. For purposes of quality<br />
control of <strong>the</strong> <strong>vacc<strong>in</strong>e</strong>, <strong>the</strong>refore, <strong>the</strong> Organi-<br />
zation had to make contractual service agree-<br />
ments with appropriate laboratories whereby<br />
<strong>the</strong>y would undertake to conduct specified<br />
tests.<br />
In December 1966 <strong>the</strong> Connaught Medical<br />
Research Laboratories, University of Toron-<br />
to, Canada, had accepted a contract from<br />
<strong>the</strong> WHO Regional Office for <strong>the</strong> Americas<br />
for <strong>the</strong> provision of technical services to<br />
improve <strong>the</strong> quality of <strong>smallpox</strong> <strong>vacc<strong>in</strong>e</strong> be<strong>in</strong>g<br />
produced by laboratories <strong>in</strong> that region (see<br />
Chapter 12). The services <strong>in</strong>volved <strong>in</strong>cluded<br />
an evaluation of exist<strong>in</strong>g <strong>smallpox</strong> <strong>vacc<strong>in</strong>e</strong><br />
production facilities <strong>and</strong> <strong>the</strong>ir personnel <strong>in</strong><br />
<strong>the</strong> region, <strong>the</strong> provision of tra<strong>in</strong><strong>in</strong>g for<br />
production personnel, <strong>and</strong> advice on <strong>the</strong><br />
selection of equipment necessary for <strong>the</strong><br />
production <strong>and</strong> test<strong>in</strong>g of <strong>smallpox</strong> <strong>vacc<strong>in</strong>e</strong>s<br />
be<strong>in</strong>g produced <strong>in</strong> <strong>the</strong> region. Dr Robert<br />
Wilson <strong>and</strong> Dr Paul Fenje, experts from <strong>the</strong><br />
Connaught Laboratories, visited production<br />
laboratories <strong>in</strong> <strong>the</strong> region, especially <strong>in</strong> South<br />
America, where 11 laboratories were produc-<br />
<strong>in</strong>g <strong>smallpox</strong> <strong>vacc<strong>in</strong>e</strong>, many of <strong>the</strong>m provid-<br />
<strong>in</strong>g: material that did not meet WHO stan-<br />
dards, as judged by <strong>the</strong> survey conducted <strong>in</strong><br />
1967.<br />
In 1967, similar arrangements were made<br />
by WHO with <strong>the</strong> National Institute of<br />
Public Health, Bilthoven, Ne<strong>the</strong>rl<strong>and</strong>s, of<br />
which <strong>the</strong> Director-General was Dr Jan<br />
Spa<strong>and</strong>er. The head of <strong>the</strong> <strong>vacc<strong>in</strong>e</strong> laboratory<br />
was Dr Anton Hekker.<br />
In 1969 <strong>the</strong> Connaught Laboratories were<br />
formally designated as <strong>the</strong> WHO Regional<br />
Reference Centre for Smallpox Vacc<strong>in</strong>e <strong>in</strong> <strong>the</strong><br />
Region of <strong>the</strong> Americas, <strong>and</strong> <strong>the</strong> National<br />
Institute of Public Health <strong>in</strong> Bilthoven as <strong>the</strong><br />
WHO International Reference Centre for<br />
Smallpox Vacc<strong>in</strong>e. The services provided by<br />
<strong>the</strong> International Reference Centre were as<br />
follows :