Netherton syndrome: Successful use of topical tacrolimus ... - Colleges
Netherton syndrome: Successful use of topical tacrolimus ... - Colleges
Netherton syndrome: Successful use of topical tacrolimus ... - Colleges
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Saif and Al-Khenaizan<br />
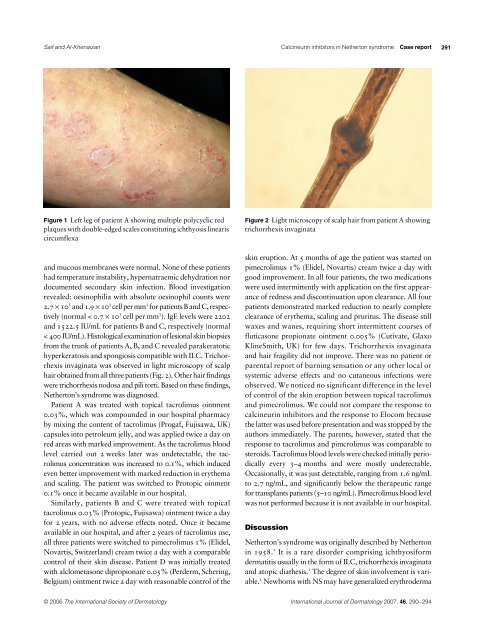
Figure 1 Left leg <strong>of</strong> patient A showing multiple polycyclic red<br />
plaques with double-edged scales constituting ichthyosis linearis<br />
circumflexa<br />
and mucous membranes were normal. None <strong>of</strong> these patients<br />
had temperature instability, hypernatraemic dehydration nor<br />
documented secondary skin infection. Blood investigation<br />
revealed: oesinophilia with absolute oesinophil counts were<br />
3<br />
3<br />
3<br />
2.7 × 10 and 1.9 × 10 cell per mm for patients B and C, respec-<br />
3<br />
3<br />
tively (normal < 0.7 × 10 cell per mm ). IgE levels were 2202<br />
and 1522.5 IU/mL for patients B and C, respectively (normal<br />
< 400 IU/mL). Histological examination <strong>of</strong> lesional skin biopsies<br />
from the trunk <strong>of</strong> patients A, B, and C revealed parakeratotic<br />
hyperkeratosis and spongiosis compatible with ILC. Trichorrhexis<br />
invaginata was observed in light microscopy <strong>of</strong> scalp<br />
hair obtained from all three patients (Fig. 2). Other hair findings<br />
were trichorrhexis nodosa and pili torti. Based on these findings,<br />
<strong>Netherton</strong>’s <strong>syndrome</strong> was diagnosed.<br />
Patient A was treated with <strong>topical</strong> <strong>tacrolimus</strong> ointment<br />
0.03%, which was compounded in our hospital pharmacy<br />
by mixing the content <strong>of</strong> <strong>tacrolimus</strong> (Progaf, Fujisawa, UK)<br />
capsules into petroleum jelly, and was applied twice a day on<br />
red areas with marked improvement. As the <strong>tacrolimus</strong> blood<br />
level carried out 2 weeks later was undetectable, the <strong>tacrolimus</strong><br />
concentration was increased to 0.1%, which induced<br />
even better improvement with marked reduction in erythema<br />
and scaling. The patient was switched to Protopic oinment<br />
0.1% once it became available in our hospital.<br />
Similarly, patients B and C were treated with <strong>topical</strong><br />
<strong>tacrolimus</strong> 0.03% (Protopic, Fujisawa) ointment twice a day<br />
for 2 years, with no adverse effects noted. Once it became<br />
available in our hospital, and after 2 years <strong>of</strong> <strong>tacrolimus</strong> <strong>use</strong>,<br />
all three patients were switched to pimecrolimus 1% (Elidel,<br />
Novartis, Switzerland) cream twice a day with a comparable<br />
control <strong>of</strong> their skin disease. Patient D was initially treated<br />
with alclometasone dipropionate 0.05% (Perderm, Schering,<br />
Belgium) ointment twice a day with reasonable control <strong>of</strong> the<br />
Calcineurin inhibitors in <strong>Netherton</strong> <strong>syndrome</strong><br />
Case report<br />
Figure 2 Light microscopy <strong>of</strong> scalp hair from patient A showing<br />
trichorrhexis invaginata<br />
skin eruption. At 5 months <strong>of</strong> age the patient was started on<br />
pimecrolimus 1% (Elidel, Novartis) cream twice a day with<br />
good improvement. In all four patients, the two medications<br />
were <strong>use</strong>d intermittently with application on the first appearance<br />
<strong>of</strong> redness and discontinuation upon clearance. All four<br />
patients demonstrated marked reduction to nearly complete<br />
clearance <strong>of</strong> erythema, scaling and pruritus. The disease still<br />
waxes and wanes, requiring short intermittent courses <strong>of</strong><br />
fluticasone propionate ointment 0.005% (Cutivate, Glaxo<br />
KlineSmith, UK) for few days. Trichorrhexis invaginata<br />
and hair fragility did not improve. There was no patient or<br />
parental report <strong>of</strong> burning sensation or any other local or<br />
systemic adverse effects and no cutaneous infections were<br />
observed. We noticed no significant difference in the level<br />
<strong>of</strong> control <strong>of</strong> the skin eruption between <strong>topical</strong> <strong>tacrolimus</strong><br />
and pimecrolimus. We could not compare the response to<br />
calcineurin inhibitors and the response to Elocom beca<strong>use</strong><br />
the latter was <strong>use</strong>d before presentation and was stopped by the<br />
authors immediately. The parents, however, stated that the<br />
response to <strong>tacrolimus</strong> and pimcrolimus was comparable to<br />
steroids. Tacrolimus blood levels were checked initially periodically<br />
every 3–4 months and were mostly undetectable.<br />
Occasionally, it was just detectable, ranging from 1.6 ng/mL<br />
to 2.7 ng/mL, and significantly below the therapeutic range<br />
for transplants patients (5–10 ng/mL). Pimecrolimus blood level<br />
was not performed beca<strong>use</strong> it is not available in our hospital.<br />
Discussion<br />
<strong>Netherton</strong>’s <strong>syndrome</strong> was originally described by <strong>Netherton</strong><br />
1<br />
in 1958. It is a rare disorder comprising ichthyosiform<br />
dermatitis usually in the form <strong>of</strong> ILC, trichorrhexis invaginata<br />
1<br />
and atopic diathesis. The degree <strong>of</strong> skin involvement is vari-<br />
2<br />
able. Newborns with NS may have generalized erythroderma<br />
© 2006 The International Society <strong>of</strong> Dermatology International Journal <strong>of</strong> Dermatology 2007, 46,<br />
290–294<br />
291





![التجربية الأولي [Read-Only] - KSU](https://img.yumpu.com/15502211/1/190x135/-read-only-ksu.jpg?quality=85)









