Writing Equations for Precipitation Reactions Precipitation reactions ...
Writing Equations for Precipitation Reactions Precipitation reactions ...
Writing Equations for Precipitation Reactions Precipitation reactions ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Use the BACK button on your browser to return to this page.<br />
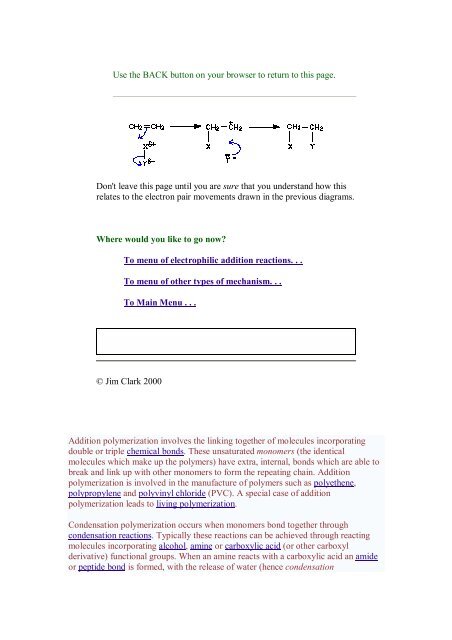
Don't leave this page until you are sure that you understand how this<br />
relates to the electron pair movements drawn in the previous diagrams.<br />
Where would you like to go now?<br />
To menu of electrophilic addition <strong>reactions</strong>. . .<br />
To menu of other types of mechanism. . .<br />
To Main Menu . . .<br />
© Jim Clark 2000<br />
Addition polymerization involves the linking together of molecules incorporating<br />
double or triple chemical bonds. These unsaturated monomers (the identical<br />
molecules which make up the polymers) have extra, internal, bonds which are able to<br />
break and link up with other monomers to <strong>for</strong>m the repeating chain. Addition<br />
polymerization is involved in the manufacture of polymers such as polyethene,<br />
polypropylene and polyvinyl chloride (PVC). A special case of addition<br />
polymerization leads to living polymerization.<br />
Condensation polymerization occurs when monomers bond together through<br />
condensation <strong>reactions</strong>. Typically these <strong>reactions</strong> can be achieved through reacting<br />
molecules incorporating alcohol, amine or carboxylic acid (or other carboxyl<br />
derivative) functional groups. When an amine reacts with a carboxylic acid an amide<br />
or peptide bond is <strong>for</strong>med, with the release of water (hence condensation