Interim Report - Hanford Site
Interim Report - Hanford Site
Interim Report - Hanford Site
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
(Absorbance × 10 5 )<br />
50<br />
40<br />
30<br />
20<br />
Abs = [(2.94 ±0.20) × 10 6 ] × 31 P Concentration<br />
10<br />
Tripoly Doublet<br />
Plot 1 Regr<br />
0<br />
0.00 0.02 0.04 0.06 0.08 0.10 0.12 0.14 0.16<br />
31 P Concentration<br />
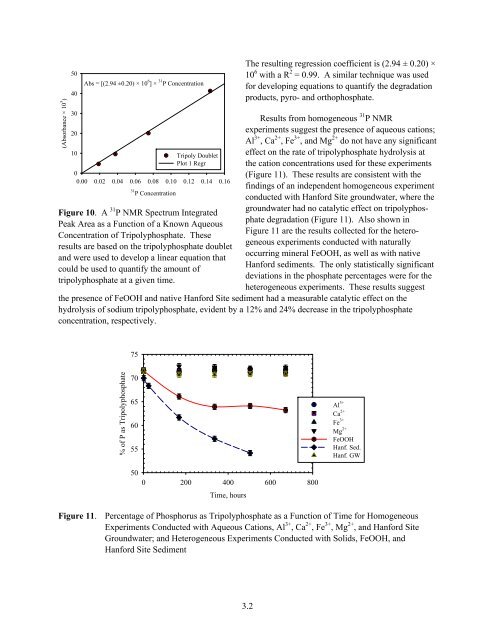
Figure 10. A 31 P NMR Spectrum Integrated<br />
Peak Area as a Function of a Known Aqueous<br />
Concentration of Tripolyphosphate. These<br />
results are based on the tripolyphosphate doublet<br />
and were used to develop a linear equation that<br />
could be used to quantify the amount of<br />
tripolyphosphate at a given time.<br />
The resulting regression coefficient is (2.94 ± 0.20) ×<br />
10 6 with a R 2 = 0.99. A similar technique was used<br />
for developing equations to quantify the degradation<br />
products, pyro- and orthophosphate.<br />
Results from homogeneous 31 P NMR<br />
experiments suggest the presence of aqueous cations;<br />
Al 3+ , Ca 2+ , Fe 3+ , and Mg 2+ do not have any significant<br />
effect on the rate of tripolyphosphate hydrolysis at<br />
the cation concentrations used for these experiments<br />
(Figure 11). These results are consistent with the<br />
findings of an independent homogeneous experiment<br />
conducted with <strong>Hanford</strong> <strong>Site</strong> groundwater, where the<br />
groundwater had no catalytic effect on tripolyphosphate<br />
degradation (Figure 11). Also shown in<br />
Figure 11 are the results collected for the heterogeneous<br />
experiments conducted with naturally<br />
occurring mineral FeOOH, as well as with native<br />
<strong>Hanford</strong> sediments. The only statistically significant<br />
deviations in the phosphate percentages were for the<br />
heterogeneous experiments. These results suggest<br />
the presence of FeOOH and native <strong>Hanford</strong> <strong>Site</strong> sediment had a measurable catalytic effect on the<br />
hydrolysis of sodium tripolyphosphate, evident by a 12% and 24% decrease in the tripolyphosphate<br />
concentration, respectively.<br />
75<br />
% of P as Tripolyphosphate<br />
70<br />
65<br />
60<br />
55<br />
Al 3+<br />
Ca 2+<br />
Fe 3+<br />
Mg 2+<br />
FeOOH<br />
Hanf. Sed.<br />
Hanf. GW<br />
50<br />
0 200 400 600 800<br />
Time, hours<br />
Figure 11. Percentage of Phosphorus as Tripolyphosphate as a Function of Time for Homogeneous<br />
Experiments Conducted with Aqueous Cations, Al 3+ , Ca 2+ , Fe 3+ , Mg 2+ , and <strong>Hanford</strong> <strong>Site</strong><br />
Groundwater; and Heterogeneous Experiments Conducted with Solids, FeOOH, and<br />
<strong>Hanford</strong> <strong>Site</strong> Sediment<br />
3.2