Interim Report - Hanford Site
Interim Report - Hanford Site
Interim Report - Hanford Site
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
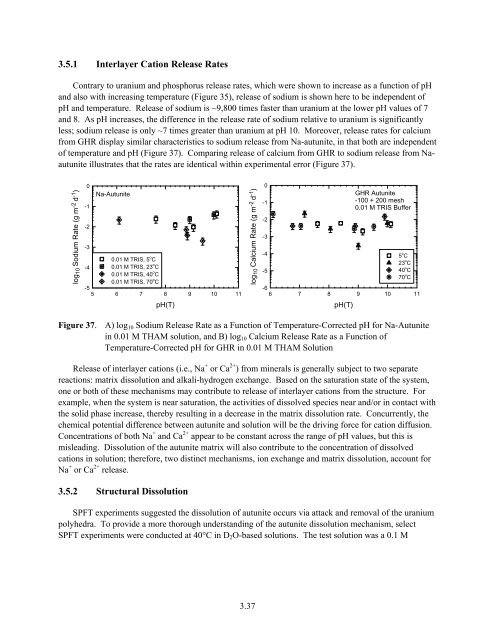
3.5.1 Interlayer Cation Release Rates<br />
Contrary to uranium and phosphorus release rates, which were shown to increase as a function of pH<br />
and also with increasing temperature (Figure 35), release of sodium is shown here to be independent of<br />
pH and temperature. Release of sodium is ~9,800 times faster than uranium at the lower pH values of 7<br />
and 8. As pH increases, the difference in the release rate of sodium relative to uranium is significantly<br />
less; sodium release is only ~7 times greater than uranium at pH 10. Moreover, release rates for calcium<br />
from GHR display similar characteristics to sodium release from Na-autunite, in that both are independent<br />
of temperature and pH (Figure 37). Comparing release of calcium from GHR to sodium release from Naautunite<br />
illustrates that the rates are identical within experimental error (Figure 37).<br />
log 10 So dium Rate (g m -2 d -1 )<br />
0<br />
-1<br />
-2<br />
-3<br />
Na-Autunite<br />
0.01 M TRIS, 5 o C<br />
-4 0.01 M TRIS, 23 o C<br />
0.01 M TRIS, 40 o C<br />
0.01 M TRIS, 70 o C<br />
-5<br />
5 6 7 8 9 10 11<br />
pH(T)<br />
log 10 Calcium Ra te (g m -2 d -1 )<br />
0<br />
-1<br />
-2<br />
-3<br />
-4 5 o C<br />
23 o C<br />
-5<br />
40 o C<br />
70 o C<br />
-6<br />
6 7 8 9 10 11<br />
pH(T)<br />
GHR Autunite<br />
-100 + 200 mesh<br />
0.01 M TRIS Buffer<br />
Figure 37. A) log 10 Sodium Release Rate as a Function of Temperature-Corre cted pH for Na-Autunite<br />
in 0.01 M THAM solution, and B) log 10 Calcium Release Rate as a Function of<br />
Temperature-Corrected pH for GHR in 0.01 M THAM Solution<br />
Release of interlayer cations (i.e., Na + or Ca 2+ ) from minerals is generally subject to two separate<br />
reactions: ma trix dissolution and alkali-hydrogen exchange. Based on the saturatio n state of the s ystem,<br />
one or both of these mecha nisms may contribute to release of interlayer cations from the structure. For<br />
exam ple, when the system is near saturation, the activities of dissolved species near and/or in contact with<br />
the solid phase increase, thereby resulting in a decrease in the matrix diss olution rate. Concurrently, the<br />
chemical potential difference between autunite and solution will be the driving force for cation diffusion.<br />
Concentrations of both Na + and Ca 2+ appear to be constant<br />
across the range of pH values, but this is<br />
misleading. Dissolution of the autunite matrix will also contribute to the concentration of dissolved<br />
cations in solution; therefore, two distinct mechanisms, ion exchange and matrix dissolution, account for<br />
Na + or Ca 2+ release.<br />
3.5.2 Structural Dissolution<br />
SPFT experiments suggested the dissolution of autunite occurs via attack and removal of the uranium<br />
polyhedra. To provide a more thorough understanding of the autunite dissolution mechanism, select<br />
SPFT experiments were conducted at 40°C in D 2 O-based solutions. The test solution was a 0.1 M<br />
3.37