Poland - IOW
Poland - IOW
Poland - IOW
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Discussion 57<br />
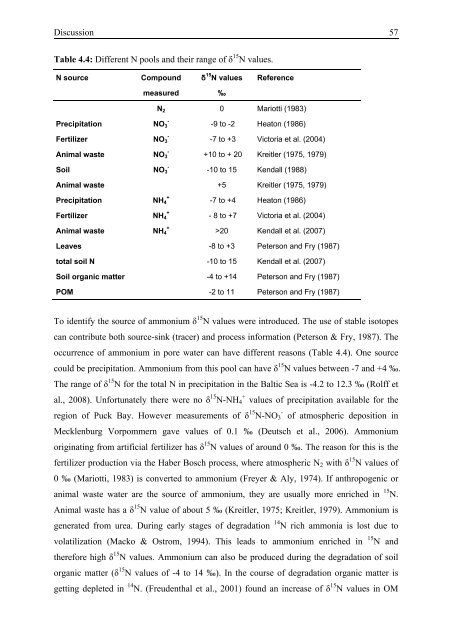
Table 4.4: Different N pools and their range of δ 15 N values.<br />
N source Compound δ 15 N values Reference<br />
measured ‰<br />
N 2 0 Mariotti (1983)<br />
Precipitation<br />
-<br />
NO 3<br />
Fertilizer<br />
-<br />
NO 3<br />
Animal waste<br />
-<br />
NO 3<br />
Soil<br />
-<br />
NO 3<br />
-9 to -2 Heaton (1986)<br />
-7 to +3 Victoria et al. (2004)<br />
+10 to + 20 Kreitler (1975, 1979)<br />
-10 to 15 Kendall (1988)<br />
Animal waste +5 Kreitler (1975, 1979)<br />
Precipitation NH 4<br />
+<br />
Fertilizer NH 4<br />
+<br />
Animal waste NH 4<br />
+<br />
-7 to +4 Heaton (1986)<br />
- 8 to +7 Victoria et al. (2004)<br />
>20 Kendall et al. (2007)<br />
Leaves -8 to +3 Peterson and Fry (1987)<br />
total soil N -10 to 15 Kendall et al. (2007)<br />
Soil organic matter -4 to +14 Peterson and Fry (1987)<br />
POM -2 to 11 Peterson and Fry (1987)<br />
To identify the source of ammonium δ 15 N values were introduced. The use of stable isotopes<br />
can contribute both source-sink (tracer) and process information (Peterson & Fry, 1987). The<br />
occurrence of ammonium in pore water can have different reasons (Table 4.4). One source<br />
could be precipitation. Ammonium from this pool can have δ 15 N values between -7 and +4 ‰.<br />
The range of δ 15 N for the total N in precipitation in the Baltic Sea is -4.2 to 12.3 ‰ (Rolff et<br />
al., 2008). Unfortunately there were no δ 15 N-NH + 4 values of precipitation available for the<br />
region of Puck Bay. However measurements of δ 15 -<br />
N-NO 3 of atmospheric deposition in<br />
Mecklenburg Vorpommern gave values of 0.1 ‰ (Deutsch et al., 2006). Ammonium<br />
originating from artificial fertilizer has δ 15 N values of around 0 ‰. The reason for this is the<br />
fertilizer production via the Haber Bosch process, where atmospheric N 2 with δ 15 N values of<br />
0 ‰ (Mariotti, 1983) is converted to ammonium (Freyer & Aly, 1974). If anthropogenic or<br />
animal waste water are the source of ammonium, they are usually more enriched in 15 N.<br />
Animal waste has a δ 15 N value of about 5 ‰ (Kreitler, 1975; Kreitler, 1979). Ammonium is<br />
generated from urea. During early stages of degradation 14 N rich ammonia is lost due to<br />
volatilization (Macko & Ostrom, 1994). This leads to ammonium enriched in 15 N and<br />
therefore high δ 15 N values. Ammonium can also be produced during the degradation of soil<br />
organic matter (δ 15 N values of -4 to 14 ‰). In the course of degradation organic matter is<br />
getting depleted in 14 N. (Freudenthal et al., 2001) found an increase of δ 15 N values in OM