CHAPTER 4. THERMODYNAMICS: THE FIRST LAW
CHAPTER 4. THERMODYNAMICS: THE FIRST LAW
CHAPTER 4. THERMODYNAMICS: THE FIRST LAW
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
4-16<br />
Ionization gaseous atom to H ion<br />
gaseous positive ion<br />
Electron gain gaseous atom to H eg<br />
Gaseous negative ion<br />
Bond dissociation gaseous molecule to H dis<br />
dissociated gaseous species<br />
G. Bond Enthalpies<br />
One consequence of the fact that bond formation in many molecules is highly localized<br />
between neighboring atoms is that bond enthalpies are fairly insensitive to the other (non-neighbor)<br />
atoms of the molecule. As a result of this, average bond enthalpies that have been determined for<br />
bonds between various atoms may be used to estimate enthalpies of reactions. Average bond<br />
enthalpies for bonds of H, C, N, and O atoms with other common elements are given in Table 4-1.<br />
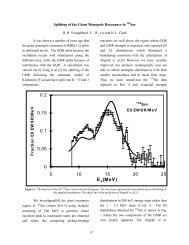
Table 4-1. Bond enthalpies of common elements (kJ/mol)<br />
H! C! C= C/ N! N= N/ O! O=<br />
H 436 413 391 463<br />
C 413 348 615 812 292 615 891 351 728<br />
N 391 292 615 891 161 418 946<br />
O 463 351 728 139 485<br />
F 563 441 270 185<br />
Si 295 290 369<br />
P 320<br />
S 339 259 477<br />
Cl 432 328 200 203<br />
Br 366 276<br />
I 299 240<br />
Bond enthalpies, such as those given in the above table, may be determined from enthalpies<br />
of formation. For example, to obtain the C!H bond enthalpy, One can use the following information<br />
for the CH molecule: CH ! C + 4H<br />
4 4(g) (g) (g)<br />
o<br />
H (CH<br />
f<br />
4(g)<br />
) = !7<strong>4.</strong>85 kJ/mol