CHAPTER 4. THERMODYNAMICS: THE FIRST LAW
CHAPTER 4. THERMODYNAMICS: THE FIRST LAW
CHAPTER 4. THERMODYNAMICS: THE FIRST LAW
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
4-18<br />
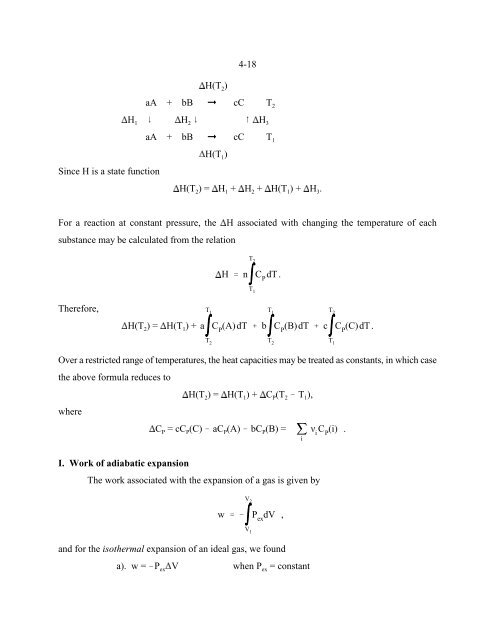
Since H is a state function<br />
H(T ) 2<br />
aA + bB ! cC T 2<br />
H 9 H 9 8 H<br />
1 2 3<br />
aA + bB ! cC T 1<br />
H(T ) 1<br />
H(T ) = H + H + H(T ) + H .<br />
2 1 2 1 3<br />
For a reaction at constant pressure, the<br />
substance may be calculated from the relation<br />
H associated with changing the temperature of each<br />
T 2<br />
H ' n C P<br />
dT.<br />
T 1<br />
Therefore, T 1<br />
T 1<br />
T 2<br />
H(T 2) = H(T 1) + a C P<br />
(A)dT % b C P<br />
(B)dT % c C P<br />
(C)dT.<br />
T 2<br />
T 2<br />
T 1<br />
Over a restricted range of temperatures, the heat capacities may be treated as constants, in which case<br />
the above formula reduces to<br />
where<br />
H(T ) = H(T ) + C (T ! T ),<br />
2 1 P 2 1<br />
C j C P (i)<br />
P = cC P(C) ! aC P(A) ! bC P(B) = .<br />
iνi<br />
I. Work of adiabatic expansion<br />
The work associated with the expansion of a gas is given by<br />
V 2<br />
w ' !<br />
V 1<br />
P ex<br />
dV<br />
,<br />
and for the isothermal expansion of an ideal gas, we found<br />
a). w = !P V<br />
ex<br />
when P = constant<br />
ex