MS20 Laboratory: Physical and Biological Factors Affecting Oxygen ...
MS20 Laboratory: Physical and Biological Factors Affecting Oxygen ...
MS20 Laboratory: Physical and Biological Factors Affecting Oxygen ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
St<strong>and</strong>ardizing the Thiosulfate Solution<br />
If we know the oxygen content of a given water sample, we can use this as our st<strong>and</strong>ard <strong>and</strong><br />
calibrate the thiosulfate solution to it. This assumes, of course, that we know the exact condition of<br />
our water sample, its salinity, temperature, <strong>and</strong> degree of oxygen saturation. If we use aerated<br />
fresh water at room temperature, we can closely approximate the actual oxygen content of the<br />
water by consulting the proper oceanographic tables.<br />
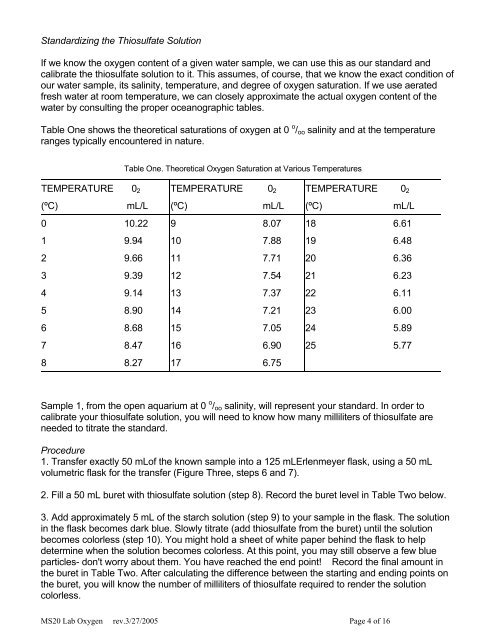
Table One shows the theoretical saturations of oxygen at 0 o / oo salinity <strong>and</strong> at the temperature<br />
ranges typically encountered in nature.<br />
Table One. Theoretical <strong>Oxygen</strong> Saturation at Various Temperatures<br />
TEMPERATURE 0 2 TEMPERATURE 0 2 TEMPERATURE 0 2<br />
(ºC) mL/L (ºC) mL/L (ºC) mL/L<br />
0 10.22 9 8.07 18 6.61<br />
1 9.94 10 7.88 19 6.48<br />
2 9.66 11 7.71 20 6.36<br />
3 9.39 12 7.54 21 6.23<br />
4 9.14 13 7.37 22 6.11<br />
5 8.90 14 7.21 23 6.00<br />
6 8.68 15 7.05 24 5.89<br />
7 8.47 16 6.90 25 5.77<br />
8 8.27 17 6.75<br />
Sample 1, from the open aquarium at 0 o / oo salinity, will represent your st<strong>and</strong>ard. In order to<br />
calibrate your thiosulfate solution, you will need to know how many milliliters of thiosulfate are<br />
needed to titrate the st<strong>and</strong>ard.<br />
Procedure<br />
1. Transfer exactly 50 mLof the known sample into a 125 mLErlenmeyer flask, using a 50 mL<br />
volumetric flask for the transfer (Figure Three, steps 6 <strong>and</strong> 7).<br />
2. Fill a 50 mL buret with thiosulfate solution (step 8). Record the buret level in Table Two below.<br />
3. Add approximately 5 mL of the starch solution (step 9) to your sample in the flask. The solution<br />
in the flask becomes dark blue. Slowly titrate (add thiosulfate from the buret) until the solution<br />
becomes colorless (step 10). You might hold a sheet of white paper behind the flask to help<br />
determine when the solution becomes colorless. At this point, you may still observe a few blue<br />
particles- don't worry about them. You have reached the end point! Record the final amount in<br />
the buret in Table Two. After calculating the difference between the starting <strong>and</strong> ending points on<br />
the buret, you will know the number of milliliters of thiosulfate required to render the solution<br />
colorless.<br />
<strong>MS20</strong> Lab <strong>Oxygen</strong> rev.3/27/2005 Page 4 of 16