Poly(2-oxazolines) in biological and biomedical application contexts
Poly(2-oxazolines) in biological and biomedical application contexts
Poly(2-oxazolines) in biological and biomedical application contexts
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1506 N. Adams, U.S. Schubert / Advanced Drug Delivery Reviews 59 (2007) 1504–1520<br />
2.1. <strong>Poly</strong>(2-oxazol<strong>in</strong>e)-based lipopolymers<br />
As discussed above, drugs are usually enclosed <strong>in</strong> a<br />
polymeric matrix or covalently attached to a polymer moiety<br />
for delivery purposes. Enclosure <strong>in</strong> a matrix either entails<br />
compound<strong>in</strong>g the drug molecule <strong>in</strong>to an amorphous polymer (as<br />
used <strong>in</strong> stents [55] <strong>and</strong> polymeric implants) or encapsulat<strong>in</strong>g it<br />
<strong>in</strong> a vehicle formed us<strong>in</strong>g amphiphilic block copolymers. These<br />
vehicles can have different organizational shapes, but all rely on<br />
the pr<strong>in</strong>ciple that amphiphiles self-assemble <strong>in</strong> water to form<br />
nanostructures composed of a hydrophobic core <strong>and</strong> a<br />
hydrophilic shell. As such, lipopolymers have received<br />
considerable attention, with the most <strong>in</strong>tensively <strong>in</strong>vestigated<br />
lipopolymers be<strong>in</strong>g poly(ethylene glycol) based moieties (PEGlipids)<br />
[56–59]. However, a number of lipo-poly<strong>oxazol<strong>in</strong>es</strong><br />
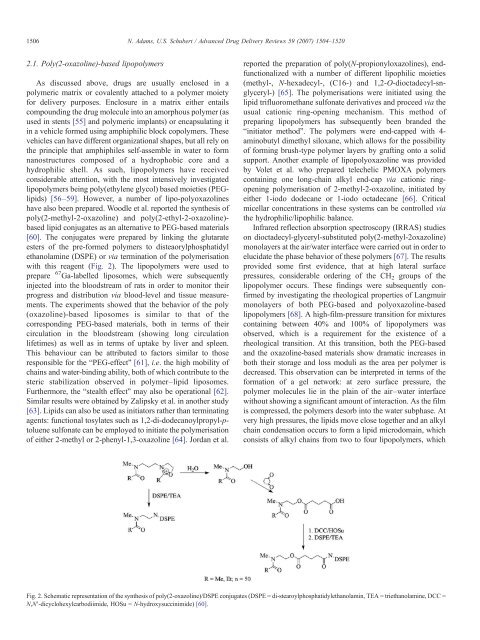
have also been prepared. Woodle et al. reported the synthesis of<br />
poly(2-methyl-2-oxazol<strong>in</strong>e) <strong>and</strong> poly(2-ethyl-2-oxazol<strong>in</strong>e)-<br />
based lipid conjugates as an alternative to PEG-based materials<br />
[60]. The conjugates were prepared by l<strong>in</strong>k<strong>in</strong>g the glutarate<br />
esters of the pre-formed polymers to disteaorylphosphatidyl<br />
ethanolam<strong>in</strong>e (DSPE) or via term<strong>in</strong>ation of the polymerisation<br />
with this reagent (Fig. 2). The lipopolymers were used to<br />
prepare 67 Ga-labelled liposomes, which were subsequently<br />
<strong>in</strong>jected <strong>in</strong>to the bloodstream of rats <strong>in</strong> order to monitor their<br />
progress <strong>and</strong> distribution via blood-level <strong>and</strong> tissue measurements.<br />
The experiments showed that the behavior of the poly<br />
(oxazol<strong>in</strong>e)-based liposomes is similar to that of the<br />
correspond<strong>in</strong>g PEG-based materials, both <strong>in</strong> terms of their<br />
circulation <strong>in</strong> the bloodstream (show<strong>in</strong>g long circulation<br />
lifetimes) as well as <strong>in</strong> terms of uptake by liver <strong>and</strong> spleen.<br />
This behaviour can be attributed to factors similar to those<br />
responsible for the “PEG-effect” [61], i.e. the high mobility of<br />
cha<strong>in</strong>s <strong>and</strong> water-b<strong>in</strong>d<strong>in</strong>g ability, both of which contribute to the<br />
steric stabilization observed <strong>in</strong> polymer–lipid liposomes.<br />
Furthermore, the “stealth effect” may also be operational [62].<br />
Similar results were obta<strong>in</strong>ed by Zalipsky et al. <strong>in</strong> another study<br />
[63]. Lipids can also be used as <strong>in</strong>itiators rather than term<strong>in</strong>at<strong>in</strong>g<br />
agents: functional tosylates such as 1,2-di-dodecanoylpropyl-ptoluene<br />
sulfonate can be employed to <strong>in</strong>itiate the polymerisation<br />
of either 2-methyl or 2-phenyl-1,3-oxazol<strong>in</strong>e [64]. Jordan et al.<br />
reported the preparation of poly(N-propionyl<strong>oxazol<strong>in</strong>es</strong>), endfunctionalized<br />
with a number of different lipophilic moieties<br />
(methyl-, N-hexadecyl-, (C16-) <strong>and</strong> 1,2-O-dioctadecyl-snglyceryl-)<br />
[65]. The polymerisations were <strong>in</strong>itiated us<strong>in</strong>g the<br />
lipid trifluoromethane sulfonate derivatives <strong>and</strong> proceed via the<br />
usual cationic r<strong>in</strong>g-open<strong>in</strong>g mechanism. This method of<br />
prepar<strong>in</strong>g lipopolymers has subsequently been br<strong>and</strong>ed the<br />
“<strong>in</strong>itiator method”. The polymers were end-capped with 4-<br />
am<strong>in</strong>obutyl dimethyl siloxane, which allows for the possibility<br />
of form<strong>in</strong>g brush-type polymer layers by graft<strong>in</strong>g onto a solid<br />
support. Another example of lipopolyoxazol<strong>in</strong>e was provided<br />
by Volet et al. who prepared telechelic PMOXA polymers<br />
conta<strong>in</strong><strong>in</strong>g one long-cha<strong>in</strong> alkyl end-cap via cationic r<strong>in</strong>gopen<strong>in</strong>g<br />
polymerisation of 2-methyl-2-oxazol<strong>in</strong>e, <strong>in</strong>itiated by<br />
either 1-iodo dodecane or 1-iodo octadecane [66]. Critical<br />
micellar concentrations <strong>in</strong> these systems can be controlled via<br />
the hydrophilic/lipophilic balance.<br />
Infrared reflection absorption spectroscopy (IRRAS) studies<br />
on dioctadecyl-glyceryl-substituted poly(2-methyl-2oxazol<strong>in</strong>e)<br />
monolayers at the air/water <strong>in</strong>terface were carried out <strong>in</strong> order to<br />
elucidate the phase behavior of these polymers [67]. The results<br />
provided some first evidence, that at high lateral surface<br />
pressures, considerable order<strong>in</strong>g of the CH 2 groups of the<br />
lipopolymer occurs. These f<strong>in</strong>d<strong>in</strong>gs were subsequently confirmed<br />
by <strong>in</strong>vestigat<strong>in</strong>g the rheological properties of Langmuir<br />
monolayers of both PEG-based <strong>and</strong> polyoxazol<strong>in</strong>e-based<br />
lipopolymers [68]. A high-film-pressure transition for mixtures<br />
conta<strong>in</strong><strong>in</strong>g between 40% <strong>and</strong> 100% of lipopolymers was<br />
observed, which is a requirement for the existence of a<br />
rheological transition. At this transition, both the PEG-based<br />
<strong>and</strong> the oxazol<strong>in</strong>e-based materials show dramatic <strong>in</strong>creases <strong>in</strong><br />
both their storage <strong>and</strong> loss moduli as the area per polymer is<br />
decreased. This observation can be <strong>in</strong>terpreted <strong>in</strong> terms of the<br />
formation of a gel network: at zero surface pressure, the<br />
polymer molecules lie <strong>in</strong> the pla<strong>in</strong> of the air–water <strong>in</strong>terface<br />
without show<strong>in</strong>g a significant amount of <strong>in</strong>teraction. As the film<br />
is compressed, the polymers desorb <strong>in</strong>to the water subphase. At<br />
very high pressures, the lipids move close together <strong>and</strong> an alkyl<br />
cha<strong>in</strong> condensation occurs to form a lipid microdoma<strong>in</strong>, which<br />
consists of alkyl cha<strong>in</strong>s from two to four lipopolymers, which<br />
Fig. 2. Schematic representation of the synthesis of poly(2-oxazol<strong>in</strong>e)/DSPE conjugates (DSPE = di-stearoylphosphatidylethanolam<strong>in</strong>, TEA = triethanolam<strong>in</strong>e, DCC =<br />
N,N′-dicyclohexylcarbodiimide, HOSu = N-hydroxysucc<strong>in</strong>imide) [60].