Poly(2-oxazolines) in biological and biomedical application contexts
Poly(2-oxazolines) in biological and biomedical application contexts
Poly(2-oxazolines) in biological and biomedical application contexts
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
N. Adams, U.S. Schubert / Advanced Drug Delivery Reviews 59 (2007) 1504–1520<br />
1507<br />
Fig. 3. Possible model to expla<strong>in</strong> network formation <strong>in</strong> lipopolymers. (A)<br />
Hydrated cha<strong>in</strong> with water molecules at locations where H-bond<strong>in</strong>g sites are<br />
exposed to bulk water, (B) hydrated water molecules act<strong>in</strong>g as <strong>in</strong>tercalates [68].<br />
do not cluster extensively. The hydrophilic part of the polymer<br />
is considered to be fully hydrated at low pressures; as the<br />
pressure is <strong>in</strong>creased, the water is squeezed out from the<br />
polymer water complex, giv<strong>in</strong>g rise to hydrogen-bonded<br />
bridges between the cha<strong>in</strong>s (Fig. 3).<br />
Nuyken <strong>and</strong> others subsequently ref<strong>in</strong>ed this picture even<br />
further by us<strong>in</strong>g X-ray <strong>and</strong> neutron reflectometry [69]. Experiments<br />
showed, that the phase transition observed at high surface<br />
pressures is <strong>in</strong>deed due to alkyl cha<strong>in</strong> condensation, triggered by<br />
stra<strong>in</strong> exerted by the poly(2-methyl-2-oxazol<strong>in</strong>e) units on the lipid<br />
moieties. The stra<strong>in</strong> orig<strong>in</strong>ates from the conf<strong>in</strong>ement of the<br />
polyoxazol<strong>in</strong>e to the <strong>in</strong>terface, which, <strong>in</strong> turn, leads to a reduction<br />
of the conformational entropy of the cha<strong>in</strong>s below that of the free<br />
cha<strong>in</strong>s. Pass<strong>in</strong>g the phase transition, the stra<strong>in</strong> leads to partial<br />
immersion of the lipids <strong>in</strong> the aqueous phase.<br />
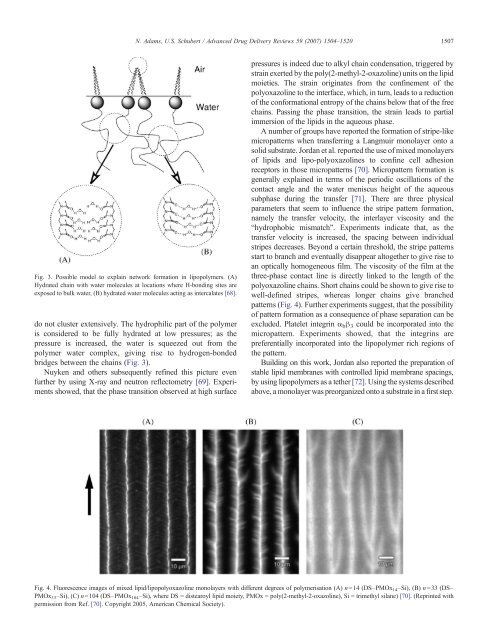
A number of groups have reported the formation of stripe-like<br />
micropatterns when transferr<strong>in</strong>g a Langmuir monolayer onto a<br />
solid substrate. Jordan et al. reported the use of mixed monolayers<br />
of lipids <strong>and</strong> lipo-poly<strong>oxazol<strong>in</strong>es</strong> to conf<strong>in</strong>e cell adhesion<br />
receptors <strong>in</strong> those micropatterns [70]. Micropatternformationis<br />
generally expla<strong>in</strong>ed <strong>in</strong> terms of the periodic oscillations of the<br />
contact angle <strong>and</strong> the water meniscus height of the aqueous<br />
subphase dur<strong>in</strong>g the transfer [71]. There are three physical<br />
parameters that seem to <strong>in</strong>fluence the stripe pattern formation,<br />
namely the transfer velocity, the <strong>in</strong>terlayer viscosity <strong>and</strong> the<br />
“hydrophobic mismatch”. Experiments <strong>in</strong>dicate that, as the<br />
transfer velocity is <strong>in</strong>creased, the spac<strong>in</strong>g between <strong>in</strong>dividual<br />
stripes decreases. Beyond a certa<strong>in</strong> threshold, the stripe patterns<br />
start to branch <strong>and</strong> eventually disappear altogether to give rise to<br />
an optically homogeneous film. The viscosity of the film at the<br />
three-phase contact l<strong>in</strong>e is directly l<strong>in</strong>ked to the length of the<br />
polyoxazol<strong>in</strong>e cha<strong>in</strong>s. Short cha<strong>in</strong>s could be shown to give rise to<br />
well-def<strong>in</strong>ed stripes, whereas longer cha<strong>in</strong>s give branched<br />
patterns (Fig. 4). Further experiments suggest, that the possibility<br />
of pattern formation as a consequence of phase separation can be<br />
excluded. Platelet <strong>in</strong>tegr<strong>in</strong> α b β 3 could be <strong>in</strong>corporated <strong>in</strong>to the<br />
micropattern. Experiments showed, that the <strong>in</strong>tegr<strong>in</strong>s are<br />
preferentially <strong>in</strong>corporated <strong>in</strong>to the lipopolymer rich regions of<br />
the pattern.<br />
Build<strong>in</strong>g on this work, Jordan also reported the preparation of<br />
stable lipid membranes with controlled lipid membrane spac<strong>in</strong>gs,<br />
by us<strong>in</strong>g lipopolymers as a tether [72]. Us<strong>in</strong>g the systems described<br />
above, a monolayer was preorganized onto a substrate <strong>in</strong> a first step.<br />
Fig. 4. Fluorescence images of mixed lipid/lipopolyoxazol<strong>in</strong>e monolayers with different degrees of polymerisation (A) n=14 (DS–PMOx 14 –Si), (B) n=33 (DS–<br />
PMOx 33 –Si), (C) n=104 (DS–PMOx 104 –Si), where DS = distearoyl lipid moiety, PMOx = poly(2-methyl-2-oxazol<strong>in</strong>e), Si = trimethyl silane) [70]. (Repr<strong>in</strong>ted with<br />
permission from Ref. [70]. Copyright 2005, American Chemical Society).