Poly(2-oxazolines) in biological and biomedical application contexts
Poly(2-oxazolines) in biological and biomedical application contexts
Poly(2-oxazolines) in biological and biomedical application contexts
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1510 N. Adams, U.S. Schubert / Advanced Drug Delivery Reviews 59 (2007) 1504–1520<br />
Fig. 7. Schematic representation of a polymer nanoreactor with por<strong>in</strong>s <strong>in</strong>corporated<br />
<strong>in</strong> the membrane <strong>and</strong> enclosed enzymes (enzymes = boxes, substrate = small circle,<br />
reaction product = small triangles) [88].<br />
homogenous sizes were obta<strong>in</strong>ed via rapid extrusion through<br />
polycarbonate filters. The capsules were stabilized by photopolymerisation<br />
of the methacrylate end-groups. Consistent with<br />
the results for the membrane system, experiments showed, that<br />
the por<strong>in</strong>s <strong>in</strong>corporated <strong>in</strong>to the capsules ma<strong>in</strong>ta<strong>in</strong>ed their<br />
activity, despite a mismatch <strong>in</strong> the hydrophilic/hydrophobic<br />
balance of the copolymer membrane system. When enzymes are<br />
<strong>in</strong>corporated <strong>in</strong>to the capsule, the rate <strong>and</strong> selectivity of<br />
substrate penetration <strong>in</strong>to the capsule <strong>and</strong> thus enzyme reaction<br />
k<strong>in</strong>etics could be controlled. This was demonstrated by<br />
<strong>in</strong>corporat<strong>in</strong>g β-lactamase <strong>in</strong>to the nanocapsules <strong>and</strong> monitor<strong>in</strong>g<br />
the rate of hydrolysis of ampicil<strong>in</strong>, which is a β-lactam<br />
antibiotic. The results <strong>in</strong>dicate, that the relative activity of the<br />
nanocapsules <strong>in</strong>creases l<strong>in</strong>early with an <strong>in</strong>creas<strong>in</strong>g concentration<br />
of prote<strong>in</strong> channels [89].<br />
A closer exam<strong>in</strong>ation of the <strong>in</strong>teraction between PMOXA–<br />
PDMS–PMOXA triblocks <strong>and</strong> lipids shows, that the formation<br />
of hybrid polymer/lipid nanocapsules is possible [90]. The<br />
formation of the composites is <strong>in</strong>dependent of the method used<br />
to prepare the nanocapsules (film hydration, dispersion,<br />
detergent removal) <strong>and</strong> the lipid distribution <strong>in</strong> the membranes<br />
is homogeneous both at low as well as high lipid/polymer ratios.<br />
Furthermore, a monomer exchange between polymersomes <strong>and</strong><br />
liposomes was observed, which, aga<strong>in</strong> can give rise to possible<br />
mixed structures <strong>and</strong> can be used to stabilize pre-formed <strong>and</strong><br />
loaded liposomes.<br />
As already <strong>in</strong>dicated <strong>in</strong> the discussion so far, the load<strong>in</strong>g of<br />
polymeric nanoconta<strong>in</strong>ers with functional molecules is of<br />
significant scientific <strong>in</strong>terest. A recent study showed, that<br />
nanoconta<strong>in</strong>ers consist<strong>in</strong>g of PMOXA–PDMS–PMOXA polymer<br />
can be charged with the small molecule fluorophore<br />
sulforhodam<strong>in</strong>e B as well as a labeled avid<strong>in</strong> [91]. Fluorescence<br />
correlation <strong>and</strong> fluorescence cross-correlation spectroscopy<br />
subsequently revealed, that the conta<strong>in</strong>ers have sizes rang<strong>in</strong>g<br />
from 140 to 172 nm <strong>and</strong> that both the dye as well as the prote<strong>in</strong><br />
could be loaded <strong>in</strong>to the conta<strong>in</strong>ers. However, the determ<strong>in</strong>ation<br />
of the load<strong>in</strong>g of the conta<strong>in</strong>ers with the small molecules is<br />
challeng<strong>in</strong>g, as a significant difference between the experimentally<br />
determ<strong>in</strong>ed <strong>and</strong> theoretically expected load<strong>in</strong>gs, was<br />
observed. This could be ascribed to self-quench<strong>in</strong>g of the dye<br />
molecules <strong>in</strong>side the conta<strong>in</strong>er. By contrast, there was a good<br />
agreement between experimental <strong>and</strong> theoretical load<strong>in</strong>gs for<br />
the avid<strong>in</strong> complex, with approximately 13 avid<strong>in</strong> molecules<br />
per sphere of 70 nm radius. Furthermore, the researchers<br />
prepared biot<strong>in</strong>ylated nanocapsules by mix<strong>in</strong>g the PMOXA–<br />
PDMS–PMOXA polymer with the biot<strong>in</strong>-substituted analogue.<br />
The <strong>in</strong>tr<strong>in</strong>sic b<strong>in</strong>d<strong>in</strong>g constant of streptavid<strong>in</strong> to the labeled<br />
nanocapsules was determ<strong>in</strong>ed to be 1.7±0.4×10 − 8 M, with<br />
approximately 1921 ±357 streptavid<strong>in</strong> molecules bound to each<br />
nanoconta<strong>in</strong>er.<br />
So far, all of the discussed vesicles were loaded dur<strong>in</strong>g the<br />
preparation/assembly phase. However, the feasibility of “postload<strong>in</strong>g”<br />
pre-formed conta<strong>in</strong>ers us<strong>in</strong>g bacteriophages has also<br />
been demonstrated [92]. Apart from facilitat<strong>in</strong>g the transport of<br />
maltose <strong>and</strong> maltodextr<strong>in</strong> across cell membranes, maltopor<strong>in</strong><br />
(LamB) also serves as a receptor for the λ-phage. When<br />
<strong>in</strong>corporated <strong>in</strong>to the walls of a PMOXA–PDMS–PMOXA<br />
nanoconta<strong>in</strong>er, the phages are clearly able to b<strong>in</strong>d to the por<strong>in</strong><br />
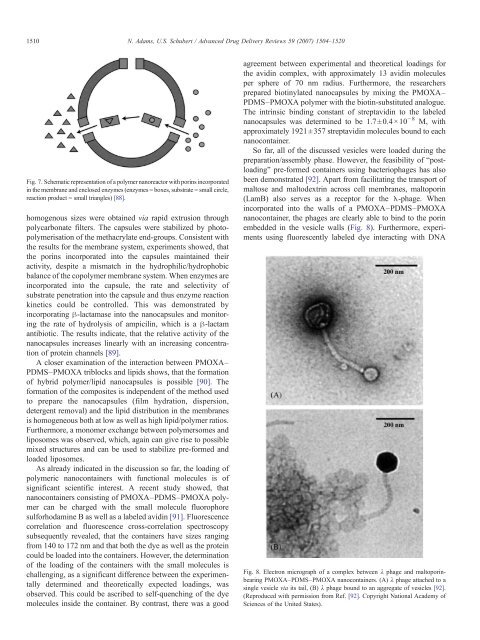
embedded <strong>in</strong> the vesicle walls (Fig. 8). Furthermore, experiments<br />
us<strong>in</strong>g fluorescently labeled dye <strong>in</strong>teract<strong>in</strong>g with DNA<br />
Fig. 8. Electron micrograph of a complex between λ phage <strong>and</strong> maltopor<strong>in</strong>bear<strong>in</strong>g<br />
PMOXA–PDMS–PMOXA nanoconta<strong>in</strong>ers. (A) λ phage attached to a<br />
s<strong>in</strong>gle vesicle via its tail, (B) λ phage bound to an aggregate of vesicles [92].<br />
(Reproduced with permission from Ref. [92]. Copyright National Academy of<br />
Sciences of the United States).