Microwave-Assisted Polymer Synthesis: Recent Developments in a ...
Microwave-Assisted Polymer Synthesis: Recent Developments in a ...
Microwave-Assisted Polymer Synthesis: Recent Developments in a ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Microwave</strong>-<strong>Assisted</strong> <strong>Polymer</strong> <strong>Synthesis</strong>: <strong>Recent</strong> <strong>Developments</strong> <strong>in</strong> ...<br />
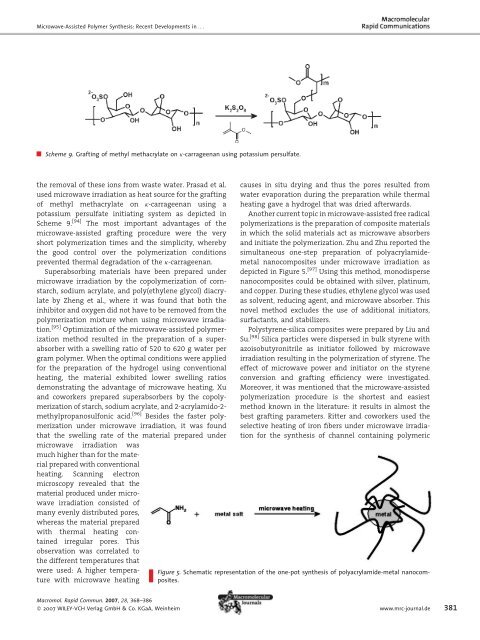
Scheme 9. Graft<strong>in</strong>g of methyl methacrylate on k-carrageenan us<strong>in</strong>g potassium persulfate.<br />
the removal of these ions from waste water. Prasad et al.<br />
used microwave irradiation as heat source for the graft<strong>in</strong>g<br />
of methyl methacrylate on k-carrageenan us<strong>in</strong>g a<br />
potassium persulfate <strong>in</strong>itiat<strong>in</strong>g system as depicted <strong>in</strong><br />
Scheme 9. [94] The most important advantages of the<br />
microwave-assisted graft<strong>in</strong>g procedure were the very<br />
short polymerization times and the simplicity, whereby<br />
the good control over the polymerization conditions<br />
prevented thermal degradation of the k-carrageenan.<br />
Superabsorb<strong>in</strong>g materials have been prepared under<br />
microwave irradiation by the copolymerization of cornstarch,<br />
sodium acrylate, and poly(ethylene glycol) diacrylate<br />
by Zheng et al., where it was found that both the<br />
<strong>in</strong>hibitor and oxygen did not have to be removed from the<br />
polymerization mixture when us<strong>in</strong>g microwave irradiation.<br />
[95] Optimization of the microwave-assisted polymerization<br />
method resulted <strong>in</strong> the preparation of a superabsorber<br />
with a swell<strong>in</strong>g ratio of 520 to 620 g water per<br />
gram polymer. When the optimal conditions were applied<br />
for the preparation of the hydrogel us<strong>in</strong>g conventional<br />
heat<strong>in</strong>g, the material exhibited lower swell<strong>in</strong>g ratios<br />
demonstrat<strong>in</strong>g the advantage of microwave heat<strong>in</strong>g. Xu<br />
and coworkers prepared superabsorbers by the copolymerization<br />
of starch, sodium acrylate, and 2-acrylamido-2-<br />
methylpropanosulfonic acid. [96] Besides the faster polymerization<br />
under microwave irradiation, it was found<br />
that the swell<strong>in</strong>g rate of the material prepared under<br />
microwave irradiation was<br />
much higher than for the material<br />
prepared with conventional<br />
heat<strong>in</strong>g. Scann<strong>in</strong>g electron<br />
microscopy revealed that the<br />
material produced under microwave<br />
irradiation consisted of<br />
many evenly distributed pores,<br />
whereas the material prepared<br />
with thermal heat<strong>in</strong>g conta<strong>in</strong>ed<br />
irregular pores. This<br />
observation was correlated to<br />
the different temperatures that<br />
were used: A higher temperature<br />
with microwave heat<strong>in</strong>g<br />
causes <strong>in</strong> situ dry<strong>in</strong>g and thus the pores resulted from<br />
water evaporation dur<strong>in</strong>g the preparation while thermal<br />
heat<strong>in</strong>g gave a hydrogel that was dried afterwards.<br />
Another current topic <strong>in</strong> microwave-assisted free radical<br />
polymerizations is the preparation of composite materials<br />
<strong>in</strong> which the solid materials act as microwave absorbers<br />
and <strong>in</strong>itiate the polymerization. Zhu and Zhu reported the<br />
simultaneous one-step preparation of polyacrylamidemetal<br />
nanocomposites under microwave irradiation as<br />
depicted <strong>in</strong> Figure 5. [97] Us<strong>in</strong>g this method, monodisperse<br />
nanocomposites could be obta<strong>in</strong>ed with silver, plat<strong>in</strong>um,<br />
and copper. Dur<strong>in</strong>g these studies, ethylene glycol was used<br />
as solvent, reduc<strong>in</strong>g agent, and microwave absorber. This<br />
novel method excludes the use of additional <strong>in</strong>itiators,<br />
surfactants, and stabilizers.<br />
Polystyrene-silica composites were prepared by Liu and<br />
Su. [98] Silica particles were dispersed <strong>in</strong> bulk styrene with<br />
azoisobutyronitrile as <strong>in</strong>itiator followed by microwave<br />
irradiation result<strong>in</strong>g <strong>in</strong> the polymerization of styrene. The<br />
effect of microwave power and <strong>in</strong>itiator on the styrene<br />
conversion and graft<strong>in</strong>g efficiency were <strong>in</strong>vestigated.<br />
Moreover, it was mentioned that the microwave-assisted<br />
polymerization procedure is the shortest and easiest<br />
method known <strong>in</strong> the literature: it results <strong>in</strong> almost the<br />
best graft<strong>in</strong>g parameters. Ritter and coworkers used the<br />
selective heat<strong>in</strong>g of iron fibers under microwave irradiation<br />
for the synthesis of channel conta<strong>in</strong><strong>in</strong>g polymeric<br />
Figure 5. Schematic representation of the one-pot synthesis of polyacrylamide-metal nanocomposites.<br />
Macromol. Rapid Commun. 2007, 28, 368–386<br />
ß 2007 WILEY-VCH Verlag GmbH & Co. KGaA, We<strong>in</strong>heim www.mrc-journal.de 381