Nanoparticles for in-vitro and in-vivo biosensing and imaging
Nanoparticles for in-vitro and in-vivo biosensing and imaging
Nanoparticles for in-vitro and in-vivo biosensing and imaging
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
38 Pr<strong>in</strong>ciples<br />
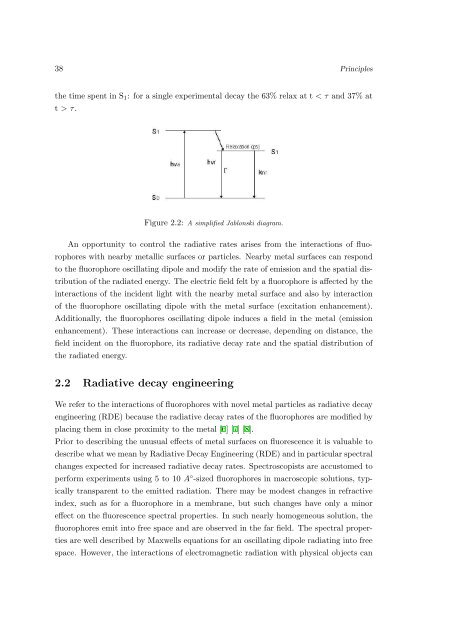
the time spent <strong>in</strong> S 1 : <strong>for</strong> a s<strong>in</strong>gle experimental decay the 63% relax at t < τ <strong>and</strong> 37% at<br />
t > τ.<br />
Figure 2.2: A simplified Jablonski diagram.<br />
An opportunity to control the radiative rates arises from the <strong>in</strong>teractions of fluorophores<br />
with nearby metallic surfaces or particles. Nearby metal surfaces can respond<br />
to the fluorophore oscillat<strong>in</strong>g dipole <strong>and</strong> modify the rate of emission <strong>and</strong> the spatial distribution<br />
of the radiated energy. The electric field felt by a fluorophore is affected by the<br />
<strong>in</strong>teractions of the <strong>in</strong>cident light with the nearby metal surface <strong>and</strong> also by <strong>in</strong>teraction<br />
of the fluorophore oscillat<strong>in</strong>g dipole with the metal surface (excitation enhancement).<br />
Additionally, the fluorophores oscillat<strong>in</strong>g dipole <strong>in</strong>duces a field <strong>in</strong> the metal (emission<br />
enhancement). These <strong>in</strong>teractions can <strong>in</strong>crease or decrease, depend<strong>in</strong>g on distance, the<br />
field <strong>in</strong>cident on the fluorophore, its radiative decay rate <strong>and</strong> the spatial distribution of<br />
the radiated energy.<br />
2.2 Radiative decay eng<strong>in</strong>eer<strong>in</strong>g<br />
We refer to the <strong>in</strong>teractions of fluorophores with novel metal particles as radiative decay<br />
eng<strong>in</strong>eer<strong>in</strong>g (RDE) because the radiative decay rates of the fluorophores are modified by<br />
plac<strong>in</strong>g them <strong>in</strong> close proximity to the metal [6] [7] [8].<br />
Prior to describ<strong>in</strong>g the unusual effects of metal surfaces on fluorescence it is valuable to<br />
describe what we mean by Radiative Decay Eng<strong>in</strong>eer<strong>in</strong>g (RDE) <strong>and</strong> <strong>in</strong> particular spectral<br />
changes expected <strong>for</strong> <strong>in</strong>creased radiative decay rates. Spectroscopists are accustomed to<br />
per<strong>for</strong>m experiments us<strong>in</strong>g 5 to 10 A ◦ -sized fluorophores <strong>in</strong> macroscopic solutions, typically<br />
transparent to the emitted radiation. There may be modest changes <strong>in</strong> refractive<br />
<strong>in</strong>dex, such as <strong>for</strong> a fluorophore <strong>in</strong> a membrane, but such changes have only a m<strong>in</strong>or<br />
effect on the fluorescence spectral properties. In such nearly homogeneous solution, the<br />
fluorophores emit <strong>in</strong>to free space <strong>and</strong> are observed <strong>in</strong> the far field. The spectral properties<br />
are well described by Maxwells equations <strong>for</strong> an oscillat<strong>in</strong>g dipole radiat<strong>in</strong>g <strong>in</strong>to free<br />
space. However, the <strong>in</strong>teractions of electromagnetic radiation with physical objects can