Philips Sonicare FlexCare - Sonicare.com - Sonicare
Philips Sonicare FlexCare - Sonicare.com - Sonicare
Philips Sonicare FlexCare - Sonicare.com - Sonicare
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
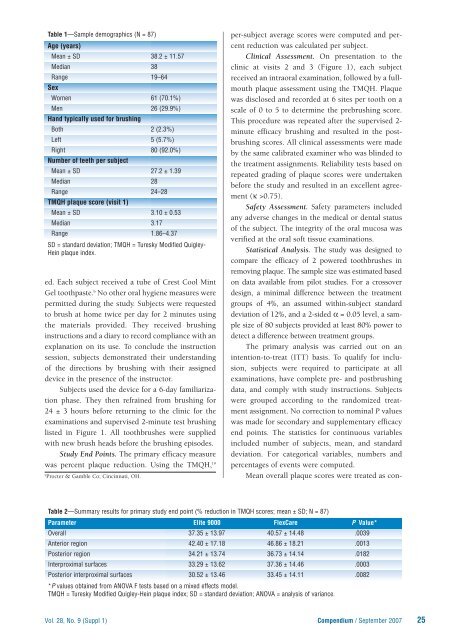
Table 1—Sample demographics (N = 87)<br />
Age (years)<br />
Mean ± SD 38.2 ± 11.57<br />
Median 38<br />
Range 19–64<br />
Sex<br />
Women 61 (70.1%)<br />
Men 26 (29.9%)<br />
Hand typically used for brushing<br />
Both 2 (2.3%)<br />
Left 5 (5.7%)<br />
Right 80 (92.0%)<br />
Number of teeth per subject<br />
Mean ± SD 27.2 ± 1.39<br />
Median 28<br />
Range 24–28<br />
TMQH plaque score (visit 1)<br />
Mean ± SD 3.10 ± 0.53<br />
Median 3.17<br />
Range 1.86–4.37<br />
SD = standard deviation; TMQH = Turesky Modified Quigley-<br />
Hein plaque index.<br />
ed. Each subject received a tube of Crest Cool Mint<br />
Gel toothpaste. b No other oral hygiene measures were<br />
permitted during the study. Subjects were requested<br />
to brush at home twice per day for 2 minutes using<br />
the materials provided. They received brushing<br />
instructions and a diary to record <strong>com</strong>pliance with an<br />
explanation on its use. To conclude the instruction<br />
session, subjects demonstrated their understanding<br />
of the directions by brushing with their assigned<br />
device in the presence of the instructor.<br />
Subjects used the device for a 6-day familiarization<br />
phase. They then refrained from brushing for<br />
24 ± 3 hours before returning to the clinic for the<br />
examinations and supervised 2-minute test brushing<br />
listed in Figure 1. All toothbrushes were supplied<br />
with new brush heads before the brushing episodes.<br />
Study End Points. The primary efficacy measure<br />
was percent plaque reduction. Using the TMQH, 19<br />
b<br />
Procter & Gamble Co; Cincinnati, OH.<br />
per-subject average scores were <strong>com</strong>puted and percent<br />
reduction was calculated per subject.<br />
Clinical Assessment. On presentation to the<br />
clinic at visits 2 and 3 (Figure 1), each subject<br />
received an intraoral examination, followed by a fullmouth<br />
plaque assessment using the TMQH. Plaque<br />
was disclosed and recorded at 6 sites per tooth on a<br />
scale of 0 to 5 to determine the prebrushing score.<br />
This procedure was repeated after the supervised 2-<br />
minute efficacy brushing and resulted in the postbrushing<br />
scores. All clinical assessments were made<br />
by the same calibrated examiner who was blinded to<br />
the treatment assignments. Reliability tests based on<br />
repeated grading of plaque scores were undertaken<br />
before the study and resulted in an excellent agreement<br />
(κ >0.75).<br />
Safety Assessment. Safety parameters included<br />
any adverse changes in the medical or dental status<br />
of the subject. The integrity of the oral mucosa was<br />
verified at the oral soft tissue examinations.<br />
Statistical Analysis. The study was designed to<br />
<strong>com</strong>pare the efficacy of 2 powered toothbrushes in<br />
removing plaque. The sample size was estimated based<br />
on data available from pilot studies. For a crossover<br />
design, a minimal difference between the treatment<br />
groups of 4%, an assumed within-subject standard<br />
deviation of 12%, and a 2-sided α = 0.05 level, a sample<br />
size of 80 subjects provided at least 80% power to<br />
detect a difference between treatment groups.<br />
The primary analysis was carried out on an<br />
intention-to-treat (ITT) basis. To qualify for inclusion,<br />
subjects were required to participate at all<br />
examinations, have <strong>com</strong>plete pre- and postbrushing<br />
data, and <strong>com</strong>ply with study instructions. Subjects<br />
were grouped according to the randomized treatment<br />
assignment. No correction to nominal P values<br />
was made for secondary and supplementary efficacy<br />
end points. The statistics for continuous variables<br />
included number of subjects, mean, and standard<br />
deviation. For categorical variables, numbers and<br />
percentages of events were <strong>com</strong>puted.<br />
Mean overall plaque scores were treated as con-<br />
Table 2—Summary results for primary study end point (% reduction in TMQH scores; mean ± SD; N = 87)<br />
Parameter Elite 9000 <strong>FlexCare</strong> P Value*<br />
Overall 37.35 ± 13.97 40.57 ± 14.48 .0039<br />
Anterior region 42.40 ± 17.18 46.86 ± 18.21 .0013<br />
Posterior region 34.21 ± 13.74 36.73 ± 14.14 .0182<br />
Interproximal surfaces 33.29 ± 13.62 37.36 ± 14.46 .0003<br />
Posterior interproximal surfaces 30.52 ± 13.46 33.45 ± 14.11 .0082<br />
*P values obtained from ANOVA F tests based on a mixed effects model.<br />
TMQH = Turesky Modified Quigley-Hein plaque index; SD = standard deviation; ANOVA = analysis of variance.<br />
Vol. 28, No. 9 (Suppl 1) Compendium / September 2007<br />
25