Philips Sonicare FlexCare - Sonicare.com - Sonicare
Philips Sonicare FlexCare - Sonicare.com - Sonicare
Philips Sonicare FlexCare - Sonicare.com - Sonicare
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
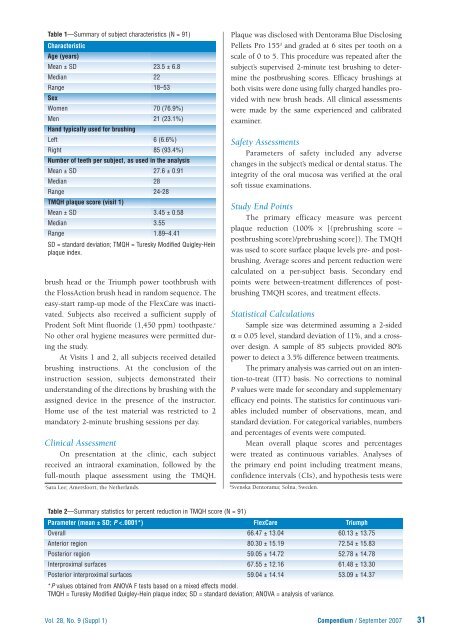
Table 1—Summary of subject characteristics (N = 91)<br />
Characteristic<br />
Age (years)<br />
Mean ± SD 23.5 ± 6.8<br />
Median 22<br />
Range 18–53<br />
Sex<br />
Women 70 (76.9%)<br />
Men 21 (23.1%)<br />
Hand typically used for brushing<br />
Left 6 (6.6%)<br />
Right 85 (93.4%)<br />
Number of teeth per subject, as used in the analysis<br />
Mean ± SD 27.6 ± 0.91<br />
Median 28<br />
Range 24-28<br />
TMQH plaque score (visit 1)<br />
Mean ± SD 3.45 ± 0.58<br />
Median 3.55<br />
Range 1.89–4.41<br />
SD = standard deviation; TMQH = Turesky Modified Quigley-Hein<br />
plaque index.<br />
brush head or the Triumph power toothbrush with<br />
the FlossAction brush head in random sequence. The<br />
easy-start ramp-up mode of the <strong>FlexCare</strong> was inactivated.<br />
Subjects also received a sufficient supply of<br />
Prodent Soft Mint fluoride (1,450 ppm) toothpaste. c<br />
No other oral hygiene measures were permitted during<br />
the study.<br />
At Visits 1 and 2, all subjects received detailed<br />
brushing instructions. At the conclusion of the<br />
instruction session, subjects demonstrated their<br />
understanding of the directions by brushing with the<br />
assigned device in the presence of the instructor.<br />
Home use of the test material was restricted to 2<br />
mandatory 2-minute brushing sessions per day.<br />
Clinical Assessment<br />
On presentation at the clinic, each subject<br />
received an intraoral examination, followed by the<br />
full-mouth plaque assessment using the TMQH.<br />
c<br />
Sara Lee; Amersfoort, the Netherlands.<br />
Plaque was disclosed with Dentorama Blue Disclosing<br />
Pellets Pro 155 d and graded at 6 sites per tooth on a<br />
scale of 0 to 5. This procedure was repeated after the<br />
subject’s supervised 2-minute test brushing to determine<br />
the postbrushing scores. Efficacy brushings at<br />
both visits were done using fully charged handles provided<br />
with new brush heads. All clinical assessments<br />
were made by the same experienced and calibrated<br />
examiner.<br />
Safety Assessments<br />
Parameters of safety included any adverse<br />
changes in the subject’s medical or dental status. The<br />
integrity of the oral mucosa was verified at the oral<br />
soft tissue examinations.<br />
Study End Points<br />
The primary efficacy measure was percent<br />
plaque reduction (100% × [(prebrushing score –<br />
postbrushing score)/prebrushing score]). The TMQH<br />
was used to score surface plaque levels pre- and postbrushing.<br />
Average scores and percent reduction were<br />
calculated on a per-subject basis. Secondary end<br />
points were between-treatment differences of postbrushing<br />
TMQH scores, and treatment effects.<br />
Statistical Calculations<br />
Sample size was determined assuming a 2-sided<br />
α = 0.05 level, standard deviation of 11%, and a crossover<br />
design. A sample of 85 subjects provided 80%<br />
power to detect a 3.5% difference between treatments.<br />
The primary analysis was carried out on an intention-to-treat<br />
(ITT) basis. No corrections to nominal<br />
P values were made for secondary and supplementary<br />
efficacy end points. The statistics for continuous variables<br />
included number of observations, mean, and<br />
standard deviation. For categorical variables, numbers<br />
and percentages of events were <strong>com</strong>puted.<br />
Mean overall plaque scores and percentages<br />
were treated as continuous variables. Analyses of<br />
the primary end point including treatment means,<br />
confidence intervals (CIs), and hypothesis tests were<br />
d<br />
Svenska Dentorama; Solna, Sweden.<br />
Table 2—Summary statistics for percent reduction in TMQH score (N = 91)<br />
Parameter (mean ± SD; P