Dynamics of glutamatergic signaling in the mushroom ... - HAL - ESPCI
Dynamics of glutamatergic signaling in the mushroom ... - HAL - ESPCI
Dynamics of glutamatergic signaling in the mushroom ... - HAL - ESPCI
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
S<strong>in</strong>akevitch et al. Neural Development 2010, 5:10<br />
http://www.neuraldevelopment.com/content/5/1/10<br />
Page 14 <strong>of</strong> 20<br />
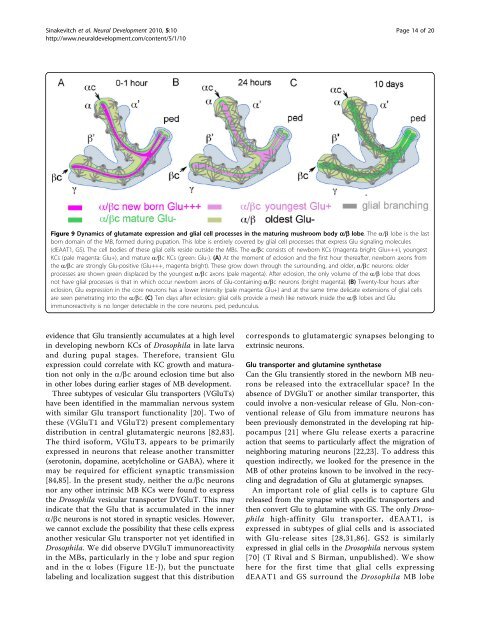
Figure 9 <strong>Dynamics</strong> <strong>of</strong> glutamate expression and glial cell processes <strong>in</strong> <strong>the</strong> matur<strong>in</strong>g <strong>mushroom</strong> body a/b lobe. The a/b lobe is <strong>the</strong> last<br />
born doma<strong>in</strong> <strong>of</strong> <strong>the</strong> MB, formed dur<strong>in</strong>g pupation. This lobe is entirely covered by glial cell processes that express Glu <strong>signal<strong>in</strong>g</strong> molecules<br />
(dEAAT1, GS). The cell bodies <strong>of</strong> <strong>the</strong>se glial cells reside outside <strong>the</strong> MBs. The a/bc consists <strong>of</strong>: newborn KCs (magenta bright: Glu+++), youngest<br />
KCs (pale magenta: Glu+), and mature a/bc KCs (green: Glu-). (A) At <strong>the</strong> moment <strong>of</strong> eclosion and <strong>the</strong> first hour <strong>the</strong>reafter, newborn axons from<br />
<strong>the</strong> a/bc are strongly Glu-positive (Glu+++, magenta bright). These grow down through <strong>the</strong> surround<strong>in</strong>g, and older, a/bc neurons: older<br />
processes are shown green displaced by <strong>the</strong> youngest a/bc axons (pale magenta). After eclosion, <strong>the</strong> only volume <strong>of</strong> <strong>the</strong> a/b lobe that does<br />
not have glial processes is that <strong>in</strong> which occur newborn axons <strong>of</strong> Glu-conta<strong>in</strong><strong>in</strong>g a/bc neurons (bright magenta). (B) Twenty-four hours after<br />
eclosion, Glu expression <strong>in</strong> <strong>the</strong> core neurons has a lower <strong>in</strong>tensity (pale magenta: Glu+) and at <strong>the</strong> same time delicate extensions <strong>of</strong> glial cells<br />
are seen penetrat<strong>in</strong>g <strong>in</strong>to <strong>the</strong> a/bc. (C) Ten days after eclosion: glial cells provide a mesh like network <strong>in</strong>side <strong>the</strong> a/b lobes and Glu<br />
immunoreactivity is no longer detectable <strong>in</strong> <strong>the</strong> core neurons. ped, pedunculus.<br />
evidence that Glu transiently accumulates at a high level<br />
<strong>in</strong> develop<strong>in</strong>g newborn KCs <strong>of</strong> Drosophila <strong>in</strong> late larva<br />
and dur<strong>in</strong>g pupal stages. Therefore, transient Glu<br />
expression could correlate with KC growth and maturation<br />
not only <strong>in</strong> <strong>the</strong> a/bc around eclosion time but also<br />
<strong>in</strong> o<strong>the</strong>r lobes dur<strong>in</strong>g earlier stages <strong>of</strong> MB development.<br />
Three subtypes <strong>of</strong> vesicular Glu transporters (VGluTs)<br />
have been identified <strong>in</strong> <strong>the</strong> mammalian nervous system<br />
with similar Glu transport functionality [20]. Two <strong>of</strong><br />
<strong>the</strong>se (VGluT1 and VGluT2) present complementary<br />
distribution <strong>in</strong> central <strong>glutamatergic</strong> neurons [82,83].<br />
The third is<strong>of</strong>orm, VGluT3, appears to be primarily<br />
expressed <strong>in</strong> neurons that release ano<strong>the</strong>r transmitter<br />
(seroton<strong>in</strong>, dopam<strong>in</strong>e, acetylchol<strong>in</strong>e or GABA), where it<br />
may be required for efficient synaptic transmission<br />
[84,85]. In <strong>the</strong> present study, nei<strong>the</strong>r <strong>the</strong> a/bc neurons<br />
nor any o<strong>the</strong>r <strong>in</strong>tr<strong>in</strong>sic MB KCs were found to express<br />
<strong>the</strong> Drosophila vesicular transporter DVGluT. This may<br />
<strong>in</strong>dicate that <strong>the</strong> Glu that is accumulated <strong>in</strong> <strong>the</strong> <strong>in</strong>ner<br />
a/bc neurons is not stored <strong>in</strong> synaptic vesicles. However,<br />
we cannot exclude <strong>the</strong> possibility that <strong>the</strong>se cells express<br />
ano<strong>the</strong>r vesicular Glu transporter not yet identified <strong>in</strong><br />
Drosophila. We did observe DVGluT immunoreactivity<br />
<strong>in</strong> <strong>the</strong> MBs, particularly <strong>in</strong> <strong>the</strong> g lobe and spur region<br />
and <strong>in</strong> <strong>the</strong> a lobes (Figure 1E-J), but <strong>the</strong> punctuate<br />
label<strong>in</strong>g and localization suggest that this distribution<br />
corresponds to <strong>glutamatergic</strong> synapses belong<strong>in</strong>g to<br />
extr<strong>in</strong>sic neurons.<br />
Glu transporter and glutam<strong>in</strong>e syn<strong>the</strong>tase<br />
Can <strong>the</strong> Glu transiently stored <strong>in</strong> <strong>the</strong> newborn MB neurons<br />
be released <strong>in</strong>to <strong>the</strong> extracellular space? In <strong>the</strong><br />
absence <strong>of</strong> DVGluT or ano<strong>the</strong>r similar transporter, this<br />
could <strong>in</strong>volve a non-vesicular release <strong>of</strong> Glu. Non-conventional<br />
release <strong>of</strong> Glu from immature neurons has<br />
been previously demonstrated <strong>in</strong> <strong>the</strong> develop<strong>in</strong>g rat hippocampus<br />
[21] where Glu release exerts a paracr<strong>in</strong>e<br />
action that seems to particularly affect <strong>the</strong> migration <strong>of</strong><br />
neighbor<strong>in</strong>g matur<strong>in</strong>g neurons [22,23]. To address this<br />
question <strong>in</strong>directly, we looked for <strong>the</strong> presence <strong>in</strong> <strong>the</strong><br />
MB <strong>of</strong> o<strong>the</strong>r prote<strong>in</strong>s known to be <strong>in</strong>volved <strong>in</strong> <strong>the</strong> recycl<strong>in</strong>g<br />
and degradation <strong>of</strong> Glu at glutamergic synapses.<br />
An important role <strong>of</strong> glial cells is to capture Glu<br />
released from <strong>the</strong> synapse with specific transporters and<br />
<strong>the</strong>n convert Glu to glutam<strong>in</strong>e with GS. The only Drosophila<br />
high-aff<strong>in</strong>ity Glu transporter, dEAAT1, is<br />
expressed <strong>in</strong> subtypes <strong>of</strong> glial cells and is associated<br />
with Glu-release sites [28,31,86]. GS2 is similarly<br />
expressed <strong>in</strong> glial cells <strong>in</strong> <strong>the</strong> Drosophila nervous system<br />
[70] (T Rival and S Birman, unpublished). We show<br />
here for <strong>the</strong> first time that glial cells express<strong>in</strong>g<br />
dEAAT1 and GS surround <strong>the</strong> Drosophila MB lobe