intensity-modulated radiotherapy: current status and issues
intensity-modulated radiotherapy: current status and issues
intensity-modulated radiotherapy: current status and issues
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
IMRT: <strong>current</strong> <strong>status</strong> <strong>and</strong> <strong>issues</strong> of interest ● IMRT CWG<br />
893<br />
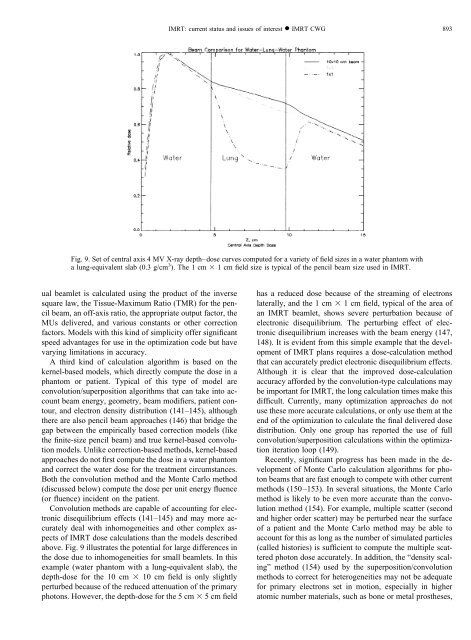
Fig. 9. Set of central axis 4 MV X-ray depth–dose curves computed for a variety of field sizes in a water phantom with<br />
a lung-equivalent slab (0.3 g/cm 3 ). The 1 cm 1cmfield size is typical of the pencil beam size used in IMRT.<br />
ual beamlet is calculated using the product of the inverse<br />
square law, the Tissue-Maximum Ratio (TMR) for the pencil<br />
beam, an off-axis ratio, the appropriate output factor, the<br />
MUs delivered, <strong>and</strong> various constants or other correction<br />
factors. Models with this kind of simplicity offer significant<br />
speed advantages for use in the optimization code but have<br />
varying limitations in accuracy.<br />
A third kind of calculation algorithm is based on the<br />
kernel-based models, which directly compute the dose in a<br />
phantom or patient. Typical of this type of model are<br />
convolution/superposition algorithms that can take into account<br />
beam energy, geometry, beam modifiers, patient contour,<br />
<strong>and</strong> electron density distribution (141–145), although<br />
there are also pencil beam approaches (146) that bridge the<br />
gap between the empirically based correction models (like<br />
the finite-size pencil beam) <strong>and</strong> true kernel-based convolution<br />
models. Unlike correction-based methods, kernel-based<br />
approaches do not first compute the dose in a water phantom<br />
<strong>and</strong> correct the water dose for the treatment circumstances.<br />
Both the convolution method <strong>and</strong> the Monte Carlo method<br />
(discussed below) compute the dose per unit energy fluence<br />
(or fluence) incident on the patient.<br />
Convolution methods are capable of accounting for electronic<br />
disequilibrium effects (141–145) <strong>and</strong> may more accurately<br />
deal with inhomogeneities <strong>and</strong> other complex aspects<br />
of IMRT dose calculations than the models described<br />
above. Fig. 9 illustrates the potential for large differences in<br />
the dose due to inhomogeneities for small beamlets. In this<br />
example (water phantom with a lung-equivalent slab), the<br />
depth-dose for the 10 cm 10 cm field is only slightly<br />
perturbed because of the reduced attenuation of the primary<br />
photons. However, the depth-dose for the 5 cm 5cmfield<br />
has a reduced dose because of the streaming of electrons<br />
laterally, <strong>and</strong> the 1 cm 1cmfield, typical of the area of<br />
an IMRT beamlet, shows severe perturbation because of<br />
electronic disequilibrium. The perturbing effect of electronic<br />
disequilibrium increases with the beam energy (147,<br />
148). It is evident from this simple example that the development<br />
of IMRT plans requires a dose-calculation method<br />
that can accurately predict electronic disequilibrium effects.<br />
Although it is clear that the improved dose-calculation<br />
accuracy afforded by the convolution-type calculations may<br />
be important for IMRT, the long calculation times make this<br />
difficult. Currently, many optimization approaches do not<br />
use these more accurate calculations, or only use them at the<br />
end of the optimization to calculate the final delivered dose<br />
distribution. Only one group has reported the use of full<br />
convolution/superposition calculations within the optimization<br />
iteration loop (149).<br />
Recently, significant progress has been made in the development<br />
of Monte Carlo calculation algorithms for photon<br />
beams that are fast enough to compete with other <strong>current</strong><br />
methods (150–153). In several situations, the Monte Carlo<br />
method is likely to be even more accurate than the convolution<br />
method (154). For example, multiple scatter (second<br />
<strong>and</strong> higher order scatter) may be perturbed near the surface<br />
of a patient <strong>and</strong> the Monte Carlo method may be able to<br />
account for this as long as the number of simulated particles<br />
(called histories) is sufficient to compute the multiple scattered<br />
photon dose accurately. In addition, the “density scaling”<br />
method (154) used by the superposition/convolution<br />
methods to correct for heterogeneities may not be adequate<br />
for primary electrons set in motion, especially in higher<br />
atomic number materials, such as bone or metal prostheses,