ENVIR215 Spring 2005 1 Lecture 2 â Basic Concepts of Energy In ...
ENVIR215 Spring 2005 1 Lecture 2 â Basic Concepts of Energy In ...
ENVIR215 Spring 2005 1 Lecture 2 â Basic Concepts of Energy In ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>ENVIR215</strong> <strong>Spring</strong> <strong>2005</strong><br />
<strong>Lecture</strong> 2 – <strong>Basic</strong> <strong>Concepts</strong> <strong>of</strong> <strong>Energy</strong><br />
<strong>In</strong> the lab, you are working on a variety <strong>of</strong> experiments that will help you to understand<br />
some basic principles <strong>of</strong> energy – measuring energy, energy conversion and storage,<br />
energy transfer, energy concentration etc.<br />
<strong>Energy</strong><br />
Scientists define energy as the capacity <strong>of</strong> an entity to do work and define work as the<br />
application <strong>of</strong> a force through a distance. We can also describe this in non-scientific<br />
terms (e.g., moving furniture).<br />
Conservation <strong>of</strong> <strong>Energy</strong><br />
<strong>Energy</strong> is neither created or destroyed; it changes from one form to another and can<br />
transfer from one bit <strong>of</strong> matter to another. The 1 st law <strong>of</strong> thermondynamics is a keystone<br />
idea in physics and can be expressed<br />
Change in internal energy = net heating (or cooling) + mechanical work done on a<br />
system<br />
Forms <strong>of</strong> <strong>Energy</strong><br />
Mechanical <strong>Energy</strong> – kinetic (motion) and potential<br />
Thermal – <strong>In</strong>ternal thermal energy (Hidden form <strong>of</strong> mechanical energy)<br />
Electrical – Electrostatic and electromagnetic<br />
Chemical – Bonds between atoms<br />
Nuclear – Based on changing the nucleus <strong>of</strong> an atom, splitting (fission) and combining<br />
(fussion) <strong>of</strong> atoms. Ultimate source <strong>of</strong> all the energy on earth (hydrogen atoms combined<br />
to make helium in the sun).<br />
Our <strong>Energy</strong> Usage<br />
Prologue <strong>of</strong> McNeil (p. 10-16) outlines a brief history <strong>of</strong> human’s energy use. The terms<br />
energy “production” and “consumption” are used to describe societies usage but these are<br />
not scientific - 1 st law <strong>of</strong> thermodynamics tells us that energy cannot be created or<br />
destroyed. Concept <strong>of</strong> energy slaves (McNeil, p. 15).<br />
1
<strong>ENVIR215</strong> <strong>Spring</strong> <strong>2005</strong><br />
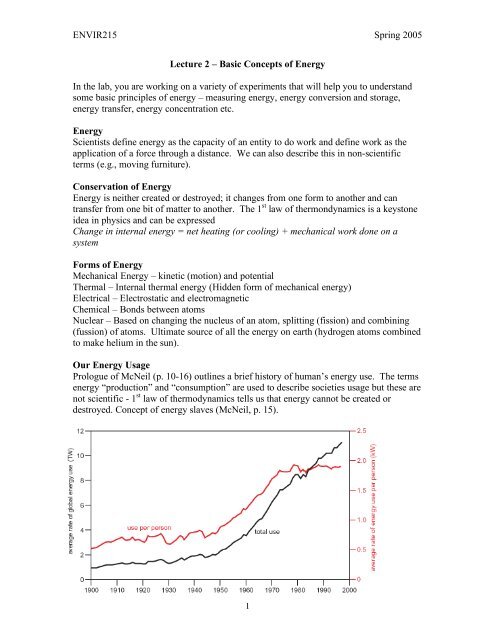
The graph above shows global energy use from 1900-2000. It was prepared in the UK by<br />
the Royal Commission on Environmental Pollution. Graphs are like this are very<br />
important in science and in society ( does anybody remember Ross Perot).<br />
Class will discuss briefly what this plot shows us.<br />
Want to look at the plot in detail. If we look at the axis labels we note that the plot has<br />
units – “TW” for the left axis and “kW”. Understanding this sort <strong>of</strong> notation is very<br />
important for this class. We want to discuss ideas we need to understand the numbers<br />
and quantities involved<br />
<strong>Energy</strong> Units<br />
We are all familiar with units <strong>of</strong> length and with the fact that we can quote lengths in<br />
different units (meters, yards, kilometers, miles, astronomical units.<br />
There are many different kinds <strong>of</strong> units for energy<br />
Scientists (at least the young ones) use the SI units (Système <strong>In</strong>ternational d'unités) <strong>of</strong><br />
Joules which is written with the shorthand J<br />
1 Joule is equal to the kinetic energy <strong>of</strong> a two-kilogram mass moving at the speed <strong>of</strong> one<br />
meter per second.<br />
We can see that the Joule is defined in terms <strong>of</strong> several other units. The units <strong>of</strong> length,<br />
time, and mass are basic units in the SI system and are defined<br />
1 meter (m) – distance traveled by light in a vacuum in 1/299,792,458 <strong>of</strong> a second.<br />
1 second (s) - the time interval equal to 9,192,631,770 periods <strong>of</strong> the radiation<br />
corresponding to the transition between the two hyperfine levels <strong>of</strong> the ground state <strong>of</strong> the<br />
cesium‐133 atom<br />
1 kilogram (kg) - defined as the mass <strong>of</strong> the <strong>In</strong>ternational Prototype Kilogram, a<br />
platinum-iridium cylinder kept at Sèvres, France, near Paris. Also the mass <strong>of</strong> 1000 cm3<br />
<strong>of</strong> water at its temperature <strong>of</strong> maximum density (39ºF).<br />
There are many other units <strong>of</strong> energy.<br />
Calorie (cal) - The amount <strong>of</strong> heat required to raise the temperature <strong>of</strong> a 1 gram <strong>of</strong> water,<br />
at or near the temperature <strong>of</strong> maximum density, one degree Celsius<br />
Food or large calories (Cal or kilocalories)<br />
Erg - <strong>Energy</strong> unit in the centimeter-gram-second cgs system<br />
BTU (British thermal unit) - the quantity <strong>of</strong> heat required to raise 1 pound <strong>of</strong> water by 1<br />
degree Fahrenheit.<br />
Electron-volt – <strong>Energy</strong> accelerating an electron through a potential difference <strong>of</strong> 1 Volt<br />
We can also think as a barrel <strong>of</strong> oil, a gallon <strong>of</strong> gasoline or a cubic foot <strong>of</strong> natural gas as<br />
units <strong>of</strong> energy<br />
2
<strong>ENVIR215</strong> <strong>Spring</strong> <strong>2005</strong><br />
Expressing very large and small quantities<br />
Often we want to describe quantities that are very small or very large relative to the basic<br />
unit.<br />
We can write small or large numbers in scientific notation e.g., the speed <strong>of</strong> light is<br />
300000000 m/s = 3 x 10 8 m/s. The spacing between atoms in a piece <strong>of</strong> copper is<br />
0.00000000023 m = 2.3 x 10 -10 m. The three numbers that appear in scientific notation<br />
are the coefficient, base which is always 10, and the exponent). Scientific notation is<br />
explained nicely at<br />
http://www.nyu.edu/pages/mathmol/textbook/scinot.html<br />
Another site <strong>of</strong> interest is<br />
http://www.powers<strong>of</strong>10.com/<br />
<strong>In</strong> SI units we can also avoid writing small or large numbers by placing prefixes before<br />
the units.<br />
Power<br />
<strong>In</strong> science, we are <strong>of</strong>ten interested in the rate at which things happen, which allows us to<br />
figure out how long things take. Rates are expressed in terms <strong>of</strong> a quantity per unit time<br />
3
<strong>ENVIR215</strong> <strong>Spring</strong> <strong>2005</strong><br />
the rate at which our car covers distance or its speed in units <strong>of</strong> length/time<br />
(meters per second or m/s)<br />
the rate at which water flows into the bath in units <strong>of</strong> volume/time (meters cubed<br />
per second or m 3 /s)<br />
The rate at which energy is generated or consumed (that is how much energy is generated<br />
or consumed per unit time) is termed the power<br />
The SI unit for power is the Watt (W) – 1 Watt is 1 Joule per second or 1 J/s<br />
There are many other units <strong>of</strong> power (e.g., horsepower (1 hp = 750 W), cal/s, foot-pound<br />
force per second).<br />
<strong>In</strong> normal conversation, we <strong>of</strong>ten use the terms power and energy almost interchangeably<br />
without thinking which one they really mean but it is important to understand the<br />
difference<br />
Unit Conversion<br />
You will come across all sorts <strong>of</strong> energy and power units in this class<br />
Just to get things really confused, one common unit for energy is the kilowatt hour (kWh)<br />
which is the energy obtained from a maintaining a power <strong>of</strong> 1000 W for 1 hour<br />
(3,600,000 J).<br />
We will give you a handout which will help you understand how to convert from 1<br />
energy unit to another. There are also handy converters available on the web. For<br />
example.<br />
http://www.convert-me.com/en/convert/energy<br />
http://www.convert-me.com/en/convert/power<br />
works well if you can put up with all the popup advertisements.<br />
(As a practical example, we can use the converter to help us compare the costs <strong>of</strong> heating<br />
a home with electricity, oil and natural gas. Heating oil costs $2/gallon and 1 gallon<br />
yields 1.5 x 10 8 J; natural gas costs $10 per 10 6 BTU; and electricity costs $0.10 per kWhr).<br />
Global <strong>Energy</strong> Use<br />
We can know go back to our original graph and we can see left hand axis has units <strong>of</strong> TW<br />
(pronounced terra Watts) which are 10 12 W or trillion Watts. The current day global<br />
energy consumption exceeds 10 13 W. The right hand axis puts that in more meaningful<br />
numbers by dividing by the world’s population. Each person uses on average a little<br />
under 2 kW = 2 x 10 3 W (equivalent to about 2 horses or 20 slaves working around the<br />
clock). <strong>In</strong> the prologue <strong>of</strong> his book McNeil talks about energy slaves. <strong>In</strong> the US our<br />
4
<strong>ENVIR215</strong> <strong>Spring</strong> <strong>2005</strong><br />
average energy use is 10 kW so we all have 100 energy slaves working for us (each slave<br />
would have to Climb Mount Rainier twice every day to average 100 W)..<br />
<strong>Energy</strong> Storage<br />
Our utilization <strong>of</strong> energy is dependent on two properties. First need to be able to store<br />
energy so that we can access it and change it to the form we need when we need it.<br />
Some sources <strong>of</strong> energy are very rich while others are poor. Nuclear energy is very rich.<br />
Chemical energy sources are also rich<br />
Hydrocarbons 30-50 kJ/g<br />
H 2<br />
145 kJ/g (but only 1/3 the energy <strong>of</strong> methane per unit<br />
volume)<br />
Foods<br />
2-17 kJ/g<br />
1 100 g candy bar provides about 1.5 x 10 6 J or 1500000 J<br />
Other sources <strong>of</strong> energy are relatively poor. For example, 1 tonne or 1000 kg <strong>of</strong> water<br />
that is elevated 10 m behind a damn contains<br />
E = mass x height x acceleration <strong>of</strong> gravity<br />
= 1000 x 10 x 10 = 10 5 J (1/15 th the energy <strong>of</strong> a candy bar)<br />
The group working in the flume will also be surprised when they calculate how much<br />
energy is stored temporarily in the moving water.<br />
Hydropower only works because such large amounts <strong>of</strong> water are involved.<br />
<strong>Energy</strong> Transmission<br />
<strong>Energy</strong> arrives at the earth via radiation <strong>of</strong> electromagnetic waves from the sun – we will<br />
talk about this more next lecture. All waves provide a means to transport energy (e.g.,<br />
ocean waves transport the energy from storms out at sea to our beaches)<br />
Heat <strong>Energy</strong> can be transmitted via conduction or by convection or the movement <strong>of</strong> fluid<br />
a process that you can look at with one <strong>of</strong> the experiments<br />
Humans transfer energy via electric transmission. Quite efficient although on average<br />
about 10% <strong>of</strong> the electricity we generate in the USA is lost is transmission and at times<br />
the fraction is even higher.<br />
Conversion <strong>of</strong> <strong>Energy</strong><br />
As many <strong>of</strong> you are experiencing in the experiments you are doing, energy can be<br />
converted from one form to another. For example in the Sterling engine chemical energy<br />
is converted to heat energy which is converted to kinetic energy and the back to heat<br />
energy as the glass balls hit the end <strong>of</strong> the test tube.<br />
One very important concept for our use <strong>of</strong> energy is efficiency.<br />
5
<strong>ENVIR215</strong> <strong>Spring</strong> <strong>2005</strong><br />
Efficiency = Amount <strong>of</strong> energy converted to a useful form / (amount converted to<br />
a useful form + the amount wasted in the form <strong>of</strong> heat). Usually expressed as an<br />
percentage.<br />
Cars + our bodies<br />
Hydrocarbon Power Plants<br />
Hydropower plants<br />
Electric motors<br />
20% efficient<br />
35% efficient<br />
90% efficient<br />
~90% efficient<br />
Our utilization <strong>of</strong> energy is <strong>of</strong>ten remarkably inefficient. For example when we drive to<br />
work.<br />
Engine 20% efficient<br />
Weight <strong>of</strong> person (75 kg) / weight <strong>of</strong> car (1500 kg) 1/20 th <strong>of</strong> total mass.<br />
99% <strong>of</strong> energy is lost.<br />
What about energy used to make the car and supply the gasoline?<br />
Amory Loving estimates our efficiency (materials and energy) is 0.02%<br />
6