Atomic structure, bonding
Atomic structure, bonding
Atomic structure, bonding
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
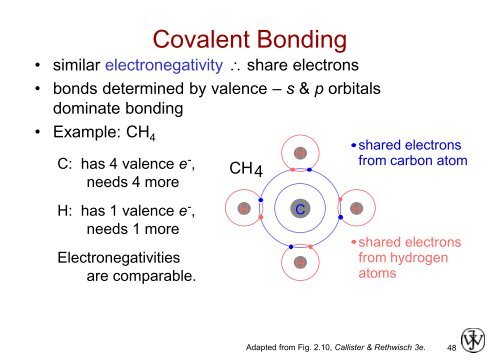
Covalent Bonding<br />
• similar electronegativity share electrons<br />
• bonds determined by valence – s & p orbitals<br />
dominate <strong>bonding</strong><br />
• Example: CH 4<br />
shared electrons<br />
C: has 4 valence e - ,<br />
needs 4 more<br />
CH 4<br />
H<br />
from carbon atom<br />
H: has 1 valence e - ,<br />
needs 1 more<br />
Electronegativities<br />
are comparable.<br />
H<br />
C<br />
H<br />
H<br />
shared electrons<br />
from hydrogen<br />
atoms<br />
Adapted from Fig. 2.10, Callister & Rethwisch 3e.<br />
48