Atomic structure, bonding
Atomic structure, bonding
Atomic structure, bonding
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
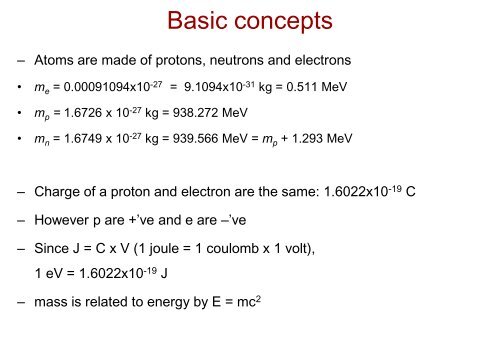
Basic concepts<br />
– Atoms are made of protons, neutrons and electrons<br />
• m e = 0.00091094x10 -27 = 9.1094x10 -31 kg = 0.511 MeV<br />
• m p = 1.6726 x 10 -27 kg = 938.272 MeV<br />
• m n = 1.6749 x 10 -27 kg = 939.566 MeV = m p + 1.293 MeV<br />
– Charge of a proton and electron are the same: 1.6022x10 -19 C<br />
– However p are +’ve and e are –’ve<br />
– Since J = C x V (1 joule = 1 coulomb x 1 volt),<br />
1 eV = 1.6022x10 -19 J<br />
– mass is related to energy by E = mc 2