CAST IRON INOCULATION - Elkem
CAST IRON INOCULATION - Elkem
CAST IRON INOCULATION - Elkem
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
After inoculation with a Ca-containing<br />
ferrosilicon, hexagonal silicate phases of<br />
the CaO•SiO 2<br />
and the CaO•Al 2<br />
O 3<br />
• 2SiO 2<br />
type will form at the surface of the existing<br />
oxide inclusions produced during<br />
nodularisation. These silicates will act<br />
as very favourable nucleation sites for<br />
graphite during solidification, due to<br />
their hexagonal crystal structure, which<br />
matches the graphite crystal lattice<br />
very well (i.e. low energy interface).<br />
Figure 5 shows a typical inclusion in<br />
ductile cast iron which is formed after<br />
nodularisation (left), and a schematic<br />
representation of the inclusion composition<br />
after inoculation (right). The surface<br />
shell contains hexagonal calcium<br />
silicates formed during inoculant addition,<br />
while the bulk particle is a product<br />
of the nodularisation treatment. Hence,<br />
the inoculation does not increase the<br />
total number of nuclei particles in the<br />
melt, but rather modifies the surface of<br />
the already existing products from nodularisation.<br />
This explains why the number<br />
density of particles in uninoculated<br />
and inoculated ductile iron melts are<br />
the same (Table 3), while the resulting<br />
nodule numbers will differ greatly due<br />
to the inclusion surface modification.<br />
When inoculation is carried out with<br />
a strontium or barium containing ferrosilicon<br />
inoculating hexagonal silicates<br />
equivalent to the calcium silicates<br />
(CaO•SiO 2<br />
and CaO•Al 2<br />
O 3<br />
•2SiO 2<br />
) will<br />
be formed (i.e. SrO•SiO 2<br />
, SrO•Al 2<br />
O 3<br />
•2SiO, BaO•SiO 2<br />
and BaO•Al 2<br />
O 3<br />
•2SiO 2<br />
)<br />
5.4 Composition of nuclei in grey iron<br />
Recent research results have identified<br />
a three step nucleation process for<br />
generating graphite flakes in grey iron.<br />
By means of electron microscope investigations,<br />
it has been revealed that<br />
a nucleus for a graphite flake consists<br />
of a particle with a body of manganeseand<br />
calcium-sulphide surrounding a<br />
nucleus core of complex Al 2<br />
O 3<br />
–XO<br />
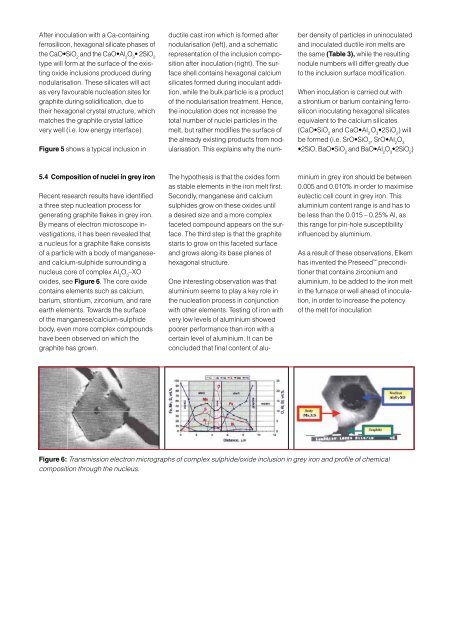
oxides, see Figure 6. The core oxide<br />
contains elements such as calcium,<br />
barium, strontium, zirconium, and rare<br />
earth elements. Towards the surface<br />
of the manganese/calcium-sulphide<br />
body, even more complex compounds<br />
have been observed on which the<br />
graphite has grown.<br />
The hypothesis is that the oxides form<br />
as stable elements in the iron melt first.<br />
Secondly, manganese and calcium<br />
sulphides grow on these oxides until<br />
a desired size and a more complex<br />
faceted compound appears on the surface.<br />
The third step is that the graphite<br />
starts to grow on this faceted surface<br />
and grows along its base planes of<br />
hexagonal structure.<br />
One interesting observation was that<br />
aluminium seems to play a key role in<br />
the nucleation process in conjunction<br />
with other elements. Testing of iron with<br />
very low levels of aluminium showed<br />
poorer performance than iron with a<br />
certain level of aluminium. It can be<br />
concluded that final content of aluminium<br />
in grey iron should be between<br />
0.005 and 0.010% in order to maximise<br />
eutectic cell count in grey iron. This<br />
aluminium content range is and has to<br />
be less than the 0.015 – 0.25% Al, as<br />
this range for pin-hole susceptibility<br />
influenced by aluminium.<br />
As a result of these observations, <strong>Elkem</strong><br />
has invented the Preseed preconditioner<br />
that contains zirconium and<br />
aluminium, to be added to the iron melt<br />
in the furnace or well ahead of inoculation,<br />
in order to increase the potency<br />
of the melt for inoculation<br />
Figure 6: Transmission electron micrographs of complex sulphide/oxide inclusion in grey iron and profile of chemical<br />
composition through the nucleus.