Report 2008-2010
Report 2008-2010
Report 2008-2010
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
37<br />
The Laboratory of Immunology and Cell Biology consists of three research<br />
groups. In <strong>2008</strong>-<strong>2010</strong>, the research was focussed to the following<br />
topics: antigenic characterization of recombinant viral and bacterial<br />
proteins, development of monoclonal antibodies (Dr. A.Žvirblienė),<br />
regulation of gene expression by alternative splicing (Dr. A.Kanopka),<br />
molecular epidemiology of tuberculosis (Dr. P.Stakėnas).<br />
Antigenicity studies of recombinant viral proteins<br />
This work was aimed at evaluating the antigenic properties of yeast-expressed<br />
recombinant proteins that might be exploited as potential<br />
vaccines or diagnostic tools. Using different immunochemical assays,<br />
the antigenic structure of yeast-expressed nucleocapsid (N) proteins<br />
of paramyxoviruses, such as human parainfluenza virus type 3 (hPIV3)<br />
and Menangle virus has been investigated. Recombinant N proteins<br />
were expressed at the Laboratory of Eukaryote Gene Engineering.<br />
The yeast-expressed N proteins used in this study were self-assembled<br />
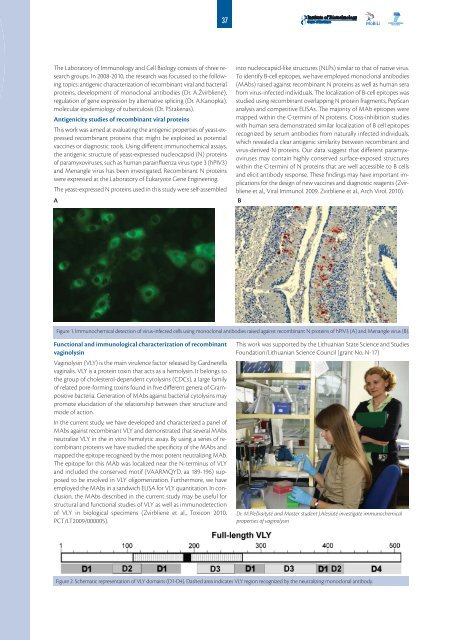
A<br />
into nucleocapsid-like structures (NLPs) similar to that of native virus.<br />
To identify B-cell epitopes, we have employed monoclonal antibodies<br />
(MAbs) raised against recombinant N proteins as well as human sera<br />
from virus-infected individuals. The localization of B-cell epitopes was<br />
studied using recombinant overlapping N protein fragments, PepScan<br />
analysis and competitive ELISAs. The majority of MAb epitopes were<br />
mapped within the C-termini of N proteins. Cross-inhibition studies<br />
with human sera demonstrated similar localization of B cell epitopes<br />
recognized by serum antibodies from naturally infected individuals,<br />
which revealed a clear antigenic similarity between recombinant and<br />
virus-derived N proteins. Our data suggest that different paramyxoviruses<br />
may contain highly conserved surface-exposed structures<br />
within the C-termini of N proteins that are well accessible to B cells<br />
and elicit antibody response. These findings may have important implications<br />
for the design of new vaccines and diagnostic reagents (Zvirbliene<br />
et al., Viral Immunol. 2009, Zvirbliene et al., Arch Virol. <strong>2010</strong>).<br />
B<br />
Figure 1. Immunochemical detection of virus-infected cells using monoclonal antibodies raised against recombinant N proteins of hPIV3 (A) and Menangle virus (B).<br />
Functional and immunological characterization of recombinant<br />
vaginolysin<br />
Vaginolysin (VLY) is the main virulence factor released by Gardnerella<br />
vaginalis. VLY is a protein toxin that acts as a hemolysin. It belongs to<br />
the group of cholesterol-dependent cytolysins (CDCs), a large family<br />
of related pore-forming toxins found in five different genera of Grampositive<br />
bacteria. Generation of MAbs against bacterial cytolysins may<br />
promote elucidation of the relationship between their structure and<br />
mode of action.<br />
In the current study, we have developed and characterized a panel of<br />
MAbs against recombinant VLY and demonstrated that several MAbs<br />
neutralize VLY in the in vitro hemolytic assay. By using a series of recombinant<br />
proteins we have studied the specificity of the MAbs and<br />
mapped the epitope recognized by the most potent neutralizing MAb.<br />
The epitope for this MAb was localized near the N-terminus of VLY<br />
and included the conserved motif (VAARMQYD, aa 189-196) supposed<br />
to be involved in VLY oligomerization. Furthermore, we have<br />
employed the MAbs in a sandwich ELISA for VLY quantitation. In conclusion,<br />
the MAbs described in the current study may be useful for<br />
structural and functional studies of VLY as well as immunodetection<br />
of VLY in biological specimens (Zvirbliene et al., Toxicon <strong>2010</strong>,<br />
PCT/LT2009/000005).<br />
This work was supported by the Lithuanian State Science and Studies<br />
Foundation/Lithuanian Science Council (grant No. N-17)<br />
Dr. M.Plečkaitytė and Master student J.Alesiūtė investigate immunochemical<br />
properties of vaginolysin<br />
Figure 2. Schematic representation of VLY domains (D1-D4). Dashed area indicates VLY region recognized by the neutralizing monoclonal antibody.