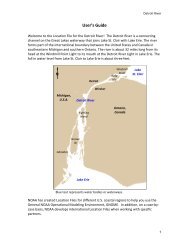
The Coastal Resource Coordinator's Bioassessment Manual
The Coastal Resource Coordinator's Bioassessment Manual
The Coastal Resource Coordinator's Bioassessment Manual
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
HAZMAT 93-1–Toxicity Tests<br />
complex wastewater effluents, similar approaches have been taken with sediment pore<br />
water or sediment elutriate. At the present time, there are no TIE procedures available to<br />
directly test bulk sediment (Ankley et al., 1992). When the cause of toxicity in bulk sediment<br />
is to be determined, TIE procedures can be conducted on sediment elutriates or pore water<br />
samples. However, toxicity of elutriate or pore water must first be confirmed. TIEs are<br />
currently only in the research and development stage.<br />
Figure 3-1. TIE strategy to evaluate contributions of contaminant groups (Ankley et<br />
al., 1991).<br />
For sediment elutriates and pore water, a phased approach to eliminate possible groups of<br />
chemicals causing toxicity can be useful (Ankley et al., 1992; Giesy and Hoke, 1990).<br />
Ammonia can be eliminated as the cause of toxicity if toxicity does not occur in samples<br />
after pH has been increased, or if measured concentrations of ammonia are known to be<br />
below toxic levels. Similarly, hydrogen sulfide is more toxic at low pH values and toxic<br />
levels of hydrogen sulfide have been identified for many species. Cationic metals can be<br />
implicated as a cause of toxicity by testing the toxicity of a chelated sample. If toxicity<br />
decreases after chelation of the sample, cationic metals are implicated and further chemical<br />
analyses of the sample may indicate which metals are responsible. Nonpolar organic<br />
compounds such as PAHs, pesticides, and PCBs are implicated as a cause if toxicity is<br />
3-15 August 1997