Introduction to Enzyme and Coenzyme Chemistry - E-Library Home
Introduction to Enzyme and Coenzyme Chemistry - E-Library Home
Introduction to Enzyme and Coenzyme Chemistry - E-Library Home
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Enzyme</strong>s are Wonderful Catalysts 35<br />
(3)<br />
O<br />
O<br />
(6)<br />
O<br />
OPh<br />
OPh<br />
OPh<br />
O<br />
O<br />
unreactive<br />
conformation<br />
O<br />
O<br />
reactive<br />
conformation<br />
O<br />
O<br />
held in reactive<br />
conformation<br />
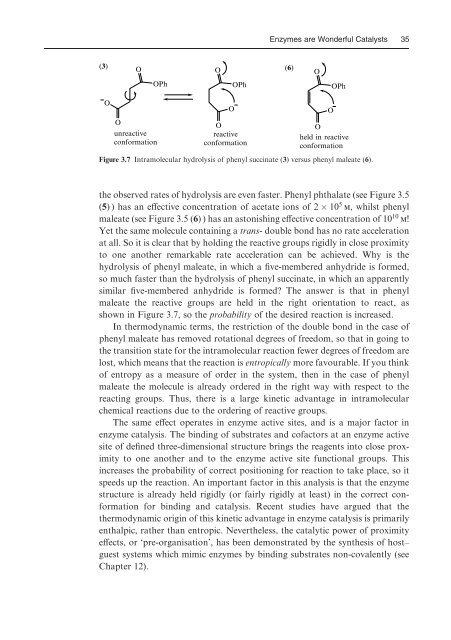
Figure 3.7 Intramolecular hydrolysis of phenyl succinate (3) versus phenyl maleate (6).<br />
the observed rates of hydrolysis are even faster. Phenyl phthalate (see Figure 3.5<br />
(5) ) has an eVective concentration of acetate ions of 2 10 5 m, whilst phenyl<br />
maleate (see Figure 3.5 (6) ) has an as<strong>to</strong>nishing eVective concentration of 10 10 m!<br />
Yet the same molecule containing a trans- double bond has no rate acceleration<br />
at all. So it is clear that by holding the reactive groups rigidly in close proximity<br />
<strong>to</strong> one another remarkable rate acceleration can be achieved. Why is the<br />
hydrolysis of phenyl maleate, in which a Wve-membered anhydride is formed,<br />
so much faster than the hydrolysis of phenyl succinate, in which an apparently<br />
similar Wve-membered anhydride is formed The answer is that in phenyl<br />
maleate the reactive groups are held in the right orientation <strong>to</strong> react, as<br />
shown in Figure 3.7, so the probability of the desired reaction is increased.<br />
In thermodynamic terms, the restriction of the double bond in the case of<br />
phenyl maleate has removed rotational degrees of freedom, so that in going <strong>to</strong><br />
the transition state for the intramolecular reaction fewer degrees of freedom are<br />
lost, which means that the reaction is entropically more favourable. If you think<br />
of entropy as a measure of order in the system, then in the case of phenyl<br />
maleate the molecule is already ordered in the right way with respect <strong>to</strong> the<br />
reacting groups. Thus, there is a large kinetic advantage in intramolecular<br />
chemical reactions due <strong>to</strong> the ordering of reactive groups.<br />
The same eVect operates in enzyme active sites, <strong>and</strong> is a major fac<strong>to</strong>r in<br />
enzyme catalysis. The binding of substrates <strong>and</strong> cofac<strong>to</strong>rs at an enzyme active<br />
site of deWned three-dimensional structure brings the reagents in<strong>to</strong> close proximity<br />
<strong>to</strong> one another <strong>and</strong> <strong>to</strong> the enzyme active site functional groups. This<br />
increases the probability of correct positioning for reaction <strong>to</strong> take place, so it<br />
speeds up the reaction. An important fac<strong>to</strong>r in this analysis is that the enzyme<br />
structure is already held rigidly (or fairly rigidly at least) in the correct conformation<br />
for binding <strong>and</strong> catalysis. Recent studies have argued that the<br />
thermodynamic origin of this kinetic advantage in enzyme catalysis is primarily<br />
enthalpic, rather than entropic. Nevertheless, the catalytic power of proximity<br />
eVects, or ‘pre-organisation’, has been demonstrated by the synthesis of host–<br />
guest systems which mimic enzymes by binding substrates non-covalently (see<br />
Chapter 12).