Safety, Quality, Efficacy: Regulating Medicines in the UK
Safety, Quality, Efficacy: Regulating Medicines in the UK
Safety, Quality, Efficacy: Regulating Medicines in the UK
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
SAFETY, QUALITY, EFFICACY: REGULATING MEDICINES IN THE <strong>UK</strong><br />
1.24 The Agency has identified that:<br />
! a potential loss of bus<strong>in</strong>ess <strong>in</strong> new active substance<br />
applications would affect <strong>in</strong>come strongly, as this<br />
work attracts <strong>the</strong> highest fees and br<strong>in</strong>gs cont<strong>in</strong>u<strong>in</strong>g<br />
<strong>in</strong>come from follow-up work;<br />
! loss of this high-profile scientific work com<strong>in</strong>g <strong>in</strong>to<br />
<strong>the</strong> Agency could make it less attractive as an<br />
employer for highly-qualified scientists;<br />
! loss of expertise could dim<strong>in</strong>ish <strong>the</strong> Agency's ability<br />
to protect <strong>UK</strong> public health through safety<br />
monitor<strong>in</strong>g and risk benefit assessment;<br />
! loss of scientific expertise could also damage<br />
<strong>the</strong> Agency's reputation and lead to fur<strong>the</strong>r loss<br />
of bus<strong>in</strong>ess.<br />
1.25 The Agency is look<strong>in</strong>g to improve <strong>the</strong> quality of <strong>the</strong><br />
services it provides to <strong>in</strong>dustry <strong>in</strong> order to attract more<br />
bus<strong>in</strong>ess under <strong>the</strong> competitive mutual recognition<br />
stream. It also aims to ma<strong>in</strong>ta<strong>in</strong> a strong presence <strong>in</strong><br />
European centralised work, and considers that <strong>the</strong><br />
changes could br<strong>in</strong>g opportunities to build on its<br />
reputation as a European leader, for example through<br />
<strong>the</strong> expanded <strong>in</strong>spection regime required by <strong>the</strong> Cl<strong>in</strong>ical<br />
Trials Directive.<br />
Volumes of work for <strong>the</strong> <strong>Medic<strong>in</strong>es</strong><br />
Control Agency are unpredictable,<br />
but generally <strong>in</strong>creas<strong>in</strong>g<br />
1.26 The number of new active substance applications<br />
submitted to <strong>the</strong> Agency and its overseas equivalents<br />
has decreased over <strong>the</strong> last seven years, reflect<strong>in</strong>g a<br />
reduction <strong>in</strong> <strong>the</strong> number of major new discoveries ready<br />
to be marketed. In o<strong>the</strong>r areas <strong>the</strong> number of<br />
applications, particularly through <strong>the</strong> European system,<br />
is <strong>in</strong>creas<strong>in</strong>g 3 , <strong>in</strong>clud<strong>in</strong>g:<br />
! renewals of market<strong>in</strong>g authorisations, which are<br />
granted for a period of five years. This process helps<br />
ensure that <strong>the</strong> licence reflects current knowledge<br />
about <strong>the</strong> balance of risks and benefits of <strong>the</strong> product.<br />
The Agency is seek<strong>in</strong>g to improve<br />
its f<strong>in</strong>ancial and operational<br />
management, by tackl<strong>in</strong>g<br />
f<strong>in</strong>ancial pressures, human<br />
resources issues and IT<br />
(a) F<strong>in</strong>ancial Management<br />
1.27 The Government established <strong>the</strong> Agency as a f<strong>in</strong>ancially<br />
self-sufficient Trad<strong>in</strong>g Fund <strong>in</strong> 1993, fully funded<br />
through fees charged to <strong>in</strong>dustry. In 2001-02 <strong>in</strong>come<br />
amounted to £40 million. The Agency is required to<br />
"break even, tak<strong>in</strong>g one year with <strong>the</strong> next, and to set its<br />
fee levels to achieve this". It may hold reasonable cash<br />
reserves, but by 1998, had accumulated a reta<strong>in</strong>ed<br />
surplus of some £17 million.<br />
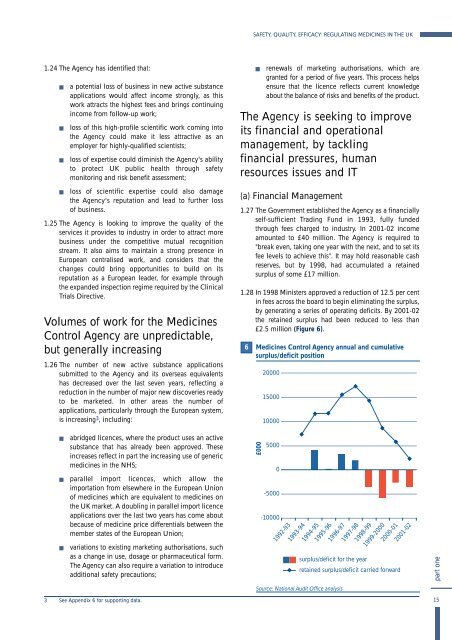
1.28 In 1998 M<strong>in</strong>isters approved a reduction of 12.5 per cent<br />
<strong>in</strong> fees across <strong>the</strong> board to beg<strong>in</strong> elim<strong>in</strong>at<strong>in</strong>g <strong>the</strong> surplus,<br />
by generat<strong>in</strong>g a series of operat<strong>in</strong>g deficits. By 2001-02<br />
<strong>the</strong> reta<strong>in</strong>ed surplus had been reduced to less than<br />
£2.5 million (Figure 6).<br />
6<br />
<strong>Medic<strong>in</strong>es</strong> Control Agency annual and cumulative<br />
surplus/deficit position<br />
20000<br />
15000<br />
10000<br />
! abridged licences, where <strong>the</strong> product uses an active<br />
substance that has already been approved. These<br />
<strong>in</strong>creases reflect <strong>in</strong> part <strong>the</strong> <strong>in</strong>creas<strong>in</strong>g use of generic<br />
medic<strong>in</strong>es <strong>in</strong> <strong>the</strong> NHS;<br />
! parallel import licences, which allow <strong>the</strong><br />
importation from elsewhere <strong>in</strong> <strong>the</strong> European Union<br />
of medic<strong>in</strong>es which are equivalent to medic<strong>in</strong>es on<br />
<strong>the</strong> <strong>UK</strong> market. A doubl<strong>in</strong>g <strong>in</strong> parallel import licence<br />
applications over <strong>the</strong> last two years has come about<br />
because of medic<strong>in</strong>e price differentials between <strong>the</strong><br />
member states of <strong>the</strong> European Union;<br />
! variations to exist<strong>in</strong>g market<strong>in</strong>g authorisations, such<br />
as a change <strong>in</strong> use, dosage or pharmaceutical form.<br />
The Agency can also require a variation to <strong>in</strong>troduce<br />
additional safety precautions;<br />
£000<br />
5000<br />
0<br />
-5000<br />
-10000<br />
1992-93<br />
1993-94<br />
1994-95<br />
1995-96<br />
1996-97<br />
1997-98<br />
1998-99<br />
1999-2000<br />
2000-01<br />
2001-02<br />
surplus/deficit for <strong>the</strong> year<br />
reta<strong>in</strong>ed surplus/deficit carried forward<br />
part one<br />
Source: National Audit Office analysis<br />
3 See Appendix 6 for support<strong>in</strong>g data.<br />
15