Safety, Quality, Efficacy: Regulating Medicines in the UK
Safety, Quality, Efficacy: Regulating Medicines in the UK
Safety, Quality, Efficacy: Regulating Medicines in the UK
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
SAFETY, QUALITY, EFFICACY: REGULATING MEDICINES IN THE <strong>UK</strong><br />
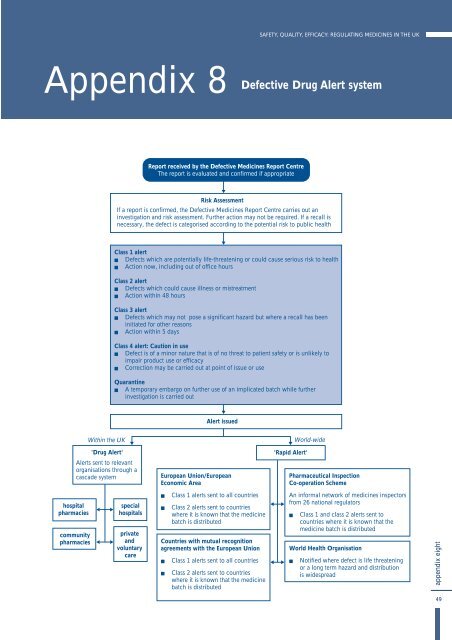
Appendix 8<br />
Defective Drug Alert system<br />
Report received by <strong>the</strong> Defective <strong>Medic<strong>in</strong>es</strong> Report Centre<br />
The report is evaluated and confirmed if appropriate<br />
Risk Assessment<br />
If a report is confirmed, <strong>the</strong> Defective <strong>Medic<strong>in</strong>es</strong> Report Centre carries out an<br />
<strong>in</strong>vestigation and risk assessment. Fur<strong>the</strong>r action may not be required. If a recall is<br />
necessary, <strong>the</strong> defect is categorised accord<strong>in</strong>g to <strong>the</strong> potential risk to public health<br />
Class 1 alert<br />
! Defects which are potentially life-threaten<strong>in</strong>g or could cause serious risk to health<br />
! Action now, <strong>in</strong>clud<strong>in</strong>g out of office hours<br />
Class 2 alert<br />
! Defects which could cause illness or mistreatment<br />
! Action with<strong>in</strong> 48 hours<br />
Class 3 alert<br />
! Defects which may not pose a significant hazard but where a recall has been<br />
<strong>in</strong>itiated for o<strong>the</strong>r reasons<br />
! Action with<strong>in</strong> 5 days<br />
Class 4 alert: Caution <strong>in</strong> use<br />
! Defect is of a m<strong>in</strong>or nature that is of no threat to patient safety or is unlikely to<br />
impair product use or efficacy<br />
! Correction may be carried out at po<strong>in</strong>t of issue or use<br />
Quarant<strong>in</strong>e<br />
! A temporary embargo on fur<strong>the</strong>r use of an implicated batch while fur<strong>the</strong>r<br />
<strong>in</strong>vestigation is carried out<br />
Alert issued<br />
With<strong>in</strong> <strong>the</strong> <strong>UK</strong><br />
'Drug Alert'<br />
Alerts sent to relevant<br />
organisations through a<br />
cascade system<br />
European Union/European<br />
Economic Area<br />
'Rapid Alert'<br />
World-wide<br />
Pharmaceutical Inspection<br />
Co-operation Scheme<br />
hospital<br />
pharmacies<br />
community<br />
pharmacies<br />
special<br />
hospitals<br />
private<br />
and<br />
voluntary<br />
care<br />
! Class 1 alerts sent to all countries<br />
! Class 2 alerts sent to countries<br />
where it is known that <strong>the</strong> medic<strong>in</strong>e<br />
batch is distributed<br />
Countries with mutual recognition<br />
agreements with <strong>the</strong> European Union<br />
! Class 1 alerts sent to all countries<br />
! Class 2 alerts sent to countries<br />
where it is known that <strong>the</strong> medic<strong>in</strong>e<br />
batch is distributed<br />
An <strong>in</strong>formal network of medic<strong>in</strong>es <strong>in</strong>spectors<br />
from 26 national regulators<br />
! Class 1 and class 2 alerts sent to<br />
countries where it is known that <strong>the</strong><br />
medic<strong>in</strong>e batch is distributed<br />
World Health Organisation<br />
! Notified where defect is life threaten<strong>in</strong>g<br />
or a long term hazard and distribution<br />
is widespread<br />
appendix eight<br />
49