Safety, Quality, Efficacy: Regulating Medicines in the UK
Safety, Quality, Efficacy: Regulating Medicines in the UK
Safety, Quality, Efficacy: Regulating Medicines in the UK
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
SAFETY, QUALITY, EFFICACY: REGULATING MEDICINES IN THE <strong>UK</strong><br />
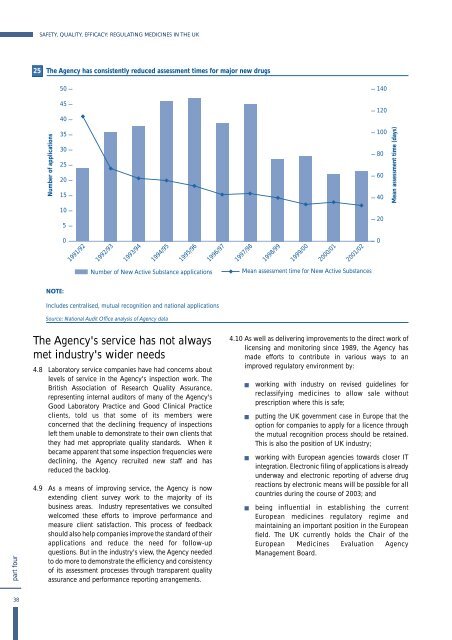
25<br />
The Agency has consistently reduced assessment times for major new drugs<br />
50<br />
45<br />
40<br />
140<br />
120<br />
Number of applications<br />
35<br />
30<br />
25<br />
20<br />
15<br />
10<br />
5<br />
100<br />
80<br />
60<br />
40<br />
20<br />
Mean assessment time (days)<br />
0<br />
1991/92<br />
1992/93<br />
1993/94<br />
1994/95<br />
1995/96<br />
1996/97<br />
1997/98<br />
1998/99<br />
1999/00<br />
2000/01<br />
2001/02<br />
0<br />
Number of New Active Substance applications<br />
Mean assessment time for New Active Substances<br />
NOTE:<br />
Includes centralised, mutual recognition and national applications<br />
Source: National Audit Office analysis of Agency data<br />
part four<br />
The Agency's service has not always<br />
met <strong>in</strong>dustry's wider needs<br />
4.8 Laboratory service companies have had concerns about<br />
levels of service <strong>in</strong> <strong>the</strong> Agency's <strong>in</strong>spection work. The<br />
British Association of Research <strong>Quality</strong> Assurance,<br />
represent<strong>in</strong>g <strong>in</strong>ternal auditors of many of <strong>the</strong> Agency's<br />
Good Laboratory Practice and Good Cl<strong>in</strong>ical Practice<br />
clients, told us that some of its members were<br />
concerned that <strong>the</strong> decl<strong>in</strong><strong>in</strong>g frequency of <strong>in</strong>spections<br />
left <strong>the</strong>m unable to demonstrate to <strong>the</strong>ir own clients that<br />
<strong>the</strong>y had met appropriate quality standards. When it<br />
became apparent that some <strong>in</strong>spection frequencies were<br />
decl<strong>in</strong><strong>in</strong>g, <strong>the</strong> Agency recruited new staff and has<br />
reduced <strong>the</strong> backlog.<br />
4.9 As a means of improv<strong>in</strong>g service, <strong>the</strong> Agency is now<br />
extend<strong>in</strong>g client survey work to <strong>the</strong> majority of its<br />
bus<strong>in</strong>ess areas. Industry representatives we consulted<br />
welcomed <strong>the</strong>se efforts to improve performance and<br />
measure client satisfaction. This process of feedback<br />
should also help companies improve <strong>the</strong> standard of <strong>the</strong>ir<br />
applications and reduce <strong>the</strong> need for follow-up<br />
questions. But <strong>in</strong> <strong>the</strong> <strong>in</strong>dustry's view, <strong>the</strong> Agency needed<br />
to do more to demonstrate <strong>the</strong> efficiency and consistency<br />
of its assessment processes through transparent quality<br />
assurance and performance report<strong>in</strong>g arrangements.<br />
4.10 As well as deliver<strong>in</strong>g improvements to <strong>the</strong> direct work of<br />
licens<strong>in</strong>g and monitor<strong>in</strong>g s<strong>in</strong>ce 1989, <strong>the</strong> Agency has<br />
made efforts to contribute <strong>in</strong> various ways to an<br />
improved regulatory environment by:<br />
! work<strong>in</strong>g with <strong>in</strong>dustry on revised guidel<strong>in</strong>es for<br />
reclassify<strong>in</strong>g medic<strong>in</strong>es to allow sale without<br />
prescription where this is safe;<br />
! putt<strong>in</strong>g <strong>the</strong> <strong>UK</strong> government case <strong>in</strong> Europe that <strong>the</strong><br />
option for companies to apply for a licence through<br />
<strong>the</strong> mutual recognition process should be reta<strong>in</strong>ed.<br />
This is also <strong>the</strong> position of <strong>UK</strong> <strong>in</strong>dustry;<br />
! work<strong>in</strong>g with European agencies towards closer IT<br />
<strong>in</strong>tegration. Electronic fil<strong>in</strong>g of applications is already<br />
underway and electronic report<strong>in</strong>g of adverse drug<br />
reactions by electronic means will be possible for all<br />
countries dur<strong>in</strong>g <strong>the</strong> course of 2003; and<br />
! be<strong>in</strong>g <strong>in</strong>fluential <strong>in</strong> establish<strong>in</strong>g <strong>the</strong> current<br />
European medic<strong>in</strong>es regulatory regime and<br />
ma<strong>in</strong>ta<strong>in</strong><strong>in</strong>g an important position <strong>in</strong> <strong>the</strong> European<br />
field. The <strong>UK</strong> currently holds <strong>the</strong> Chair of <strong>the</strong><br />
European <strong>Medic<strong>in</strong>es</strong> Evaluation Agency<br />
Management Board.<br />
38