and the Efficacy of Pit Latrine Additives - Water Research Commission
and the Efficacy of Pit Latrine Additives - Water Research Commission
and the Efficacy of Pit Latrine Additives - Water Research Commission
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
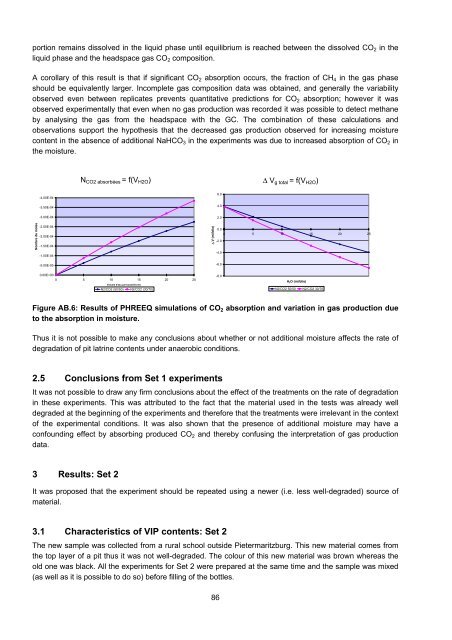
portion remains dissolved in <strong>the</strong> liquid phase until equilibrium is reached between <strong>the</strong> dissolved CO 2 in <strong>the</strong><br />
liquid phase <strong>and</strong> <strong>the</strong> headspace gas CO 2 composition.<br />
A corollary <strong>of</strong> this result is that if significant CO 2 absorption occurs, <strong>the</strong> fraction <strong>of</strong> CH 4 in <strong>the</strong> gas phase<br />
should be equivalently larger. Incomplete gas composition data was obtained, <strong>and</strong> generally <strong>the</strong> variability<br />
observed even between replicates prevents quantitative predictions for CO 2 absorption; however it was<br />
observed experimentally that even when no gas production was recorded it was possible to detect methane<br />
by analysing <strong>the</strong> gas from <strong>the</strong> headspace with <strong>the</strong> GC. The combination <strong>of</strong> <strong>the</strong>se calculations <strong>and</strong><br />
observations support <strong>the</strong> hypo<strong>the</strong>sis that <strong>the</strong> decreased gas production observed for increasing moisture<br />
content in <strong>the</strong> absence <strong>of</strong> additional NaHCO 3 in <strong>the</strong> experiments was due to increased absorption <strong>of</strong> CO 2 in<br />
<strong>the</strong> moisture.<br />
N CO2 absorbées = f(V H2O ) V g total = f(V H2O )<br />
-4.00E-04<br />
6.0<br />
-3.50E-04<br />
4.0<br />
-3.00E-04<br />
2.0<br />
Nombre de moles<br />
-2.50E-04<br />
-2.00E-04<br />
-1.50E-04<br />
V (ml/btle)<br />
0.0<br />
0 5 10 15 20 25<br />
-2.0<br />
-1.00E-04<br />
-4.0<br />
-5.00E-05<br />
-6.0<br />
0.00E+00<br />
0 5 10 15 20 25<br />
Volume d'eau par bouteille (ml)<br />
N2/CO2 (50/50) N2/CO2 (30/70)<br />
-8.0<br />
H 2O (ml/btle)<br />
N2/CO2 50/50 N2/CO2 30/70<br />
Figure AB.6: Results <strong>of</strong> PHREEQ simulations <strong>of</strong> CO 2 absorption <strong>and</strong> variation in gas production due<br />
to <strong>the</strong> absorption in moisture.<br />
Thus it is not possible to make any conclusions about whe<strong>the</strong>r or not additional moisture affects <strong>the</strong> rate <strong>of</strong><br />
degradation <strong>of</strong> pit latrine contents under anaerobic conditions.<br />
2.5 Conclusions from Set 1 experiments<br />
It was not possible to draw any firm conclusions about <strong>the</strong> effect <strong>of</strong> <strong>the</strong> treatments on <strong>the</strong> rate <strong>of</strong> degradation<br />
in <strong>the</strong>se experiments. This was attributed to <strong>the</strong> fact that <strong>the</strong> material used in <strong>the</strong> tests was already well<br />
degraded at <strong>the</strong> beginning <strong>of</strong> <strong>the</strong> experiments <strong>and</strong> <strong>the</strong>refore that <strong>the</strong> treatments were irrelevant in <strong>the</strong> context<br />
<strong>of</strong> <strong>the</strong> experimental conditions. It was also shown that <strong>the</strong> presence <strong>of</strong> additional moisture may have a<br />
confounding effect by absorbing produced CO 2 <strong>and</strong> <strong>the</strong>reby confusing <strong>the</strong> interpretation <strong>of</strong> gas production<br />
data.<br />
3 Results: Set 2<br />
It was proposed that <strong>the</strong> experiment should be repeated using a newer (i.e. less well-degraded) source <strong>of</strong><br />
material.<br />
3.1 Characteristics <strong>of</strong> VIP contents: Set 2<br />
The new sample was collected from a rural school outside Pietermaritzburg. This new material comes from<br />
<strong>the</strong> top layer <strong>of</strong> a pit thus it was not well-degraded. The colour <strong>of</strong> this new material was brown whereas <strong>the</strong><br />
old one was black. All <strong>the</strong> experiments for Set 2 were prepared at <strong>the</strong> same time <strong>and</strong> <strong>the</strong> sample was mixed<br />
(as well as it is possible to do so) before filling <strong>of</strong> <strong>the</strong> bottles.<br />
86