(BFAD) CITIZEN'S CHARTER - fda.gov.ph
(BFAD) CITIZEN'S CHARTER - fda.gov.ph
(BFAD) CITIZEN'S CHARTER - fda.gov.ph
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
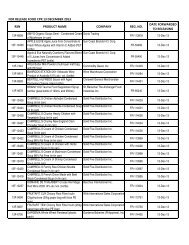
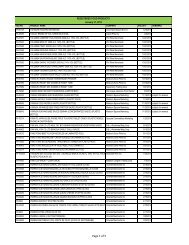
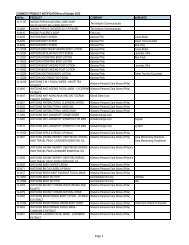
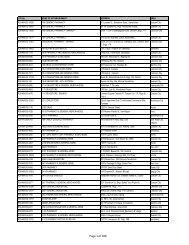
Drug Section<br />
Duration : 12 months (Calendar days)<br />
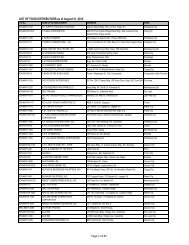
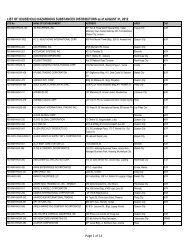
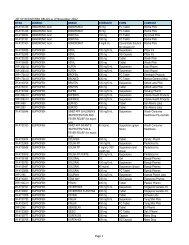
Flowchart for Drug Product Application – Initial Registration<br />
No. CLIENT/APPLICANT<br />
AGENCY ACTIVITIES PERSON LOCATION<br />
ACTION<br />
RESPONSIBLE OFFICE<br />
1 Download checklist of<br />
Client & Coop Website &<br />
requirements for product<br />
Staff<br />
<strong>BFAD</strong><br />
registration from the <strong>BFAD</strong><br />
website (www.bfad.<strong>gov</strong>.<strong>ph</strong>) or<br />
<strong>ph</strong>otocopy the same at the<br />
<strong>BFAD</strong> COOP Canteen (with<br />
fee)<br />
canteen<br />
2 Get number to the guard on- Issuance of priority number Guard PSD (Annex<br />
duty for filing of drug product<br />
evaluation. Wait for the<br />
number to be called<br />
bldg.)<br />
3 Submit product application to<br />
the evaluator on duty for<br />
assessment<br />
*For walk-in clients 1-3 steps<br />
will no longer be applicable<br />
4 Go to Accounting Section for<br />
validation of payment and pay<br />
the required fee<br />
5 Receiving & assigning Routine<br />
Slip Number (RSN) #<br />
Encoding of application for<br />
product registration<br />
6 Follow-up after 90 days<br />
proceed to the Releasing<br />
Window and check if<br />
NOD/CPR/LOD is available<br />
7 Proceed to PSD and submit<br />
compliances to NOD/re- apply<br />
for LOD<br />
8 Proceed to PAICS and submit<br />
the documents<br />
9 Proceed to releasing window<br />
and present ID/ authorization<br />
letter<br />
Pre-assess the product<br />
application & indicate the<br />
amount to be paid on the<br />
assessment slip<br />
Validation of order of<br />
payment & acceptance of<br />
payment<br />
Received /assigned RSN No.<br />
Encode the product<br />
application<br />
Check and release the Letter<br />
of Denial, Notice of<br />
Defficiency, Certificate of<br />
Product Registration (if<br />
available)<br />
Check the submitted<br />
compliance and return the<br />
documents to client for<br />
submission to PAICS. Advise<br />
client to follow-up after 60<br />
days<br />
Receive the documents<br />
encode and assign RSN.<br />
Forward the compliances to<br />
PSD for final evaluation<br />
Check and release the CPR/<br />
LOD<br />
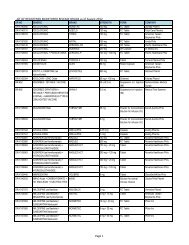
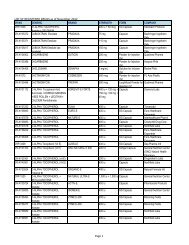
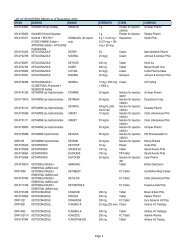
4<br />
Evaluator<br />
(FDROs)<br />
Accounting<br />
Clerk/Cashier<br />
Clerk<br />
PSD (Annex<br />
bldg.)<br />
Accounting<br />
(Rm. 113) &<br />
Cashier<br />
Section<br />
(Rm.112)<br />
Clerk PAICS (Rm.<br />
101)<br />
Releasing<br />
Personnel<br />
Releasing<br />
Window<br />
Evaluator PSD (Annex<br />
bldg.)<br />
PAICS Personnel PAICS (Rm.<br />
101)<br />
Releasing<br />
Personnel<br />
Releasing<br />
Window<br />
DURATION<br />
OF ACTION<br />
2 hours<br />
(Depending<br />
on the<br />
number of<br />
applicants)<br />
30 minutes<br />
10 minutes<br />
15 minutes<br />
90 Days<br />
(follow-up)<br />
90 days for<br />
compliance<br />
15 minutes<br />
(checking)<br />
7 months<br />
(queuing<br />
time prior to<br />
evaluation)<br />
30 minutes<br />
(checking)<br />
60 days<br />
(follow-up)<br />
30 minutes<br />
10 minutes