You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
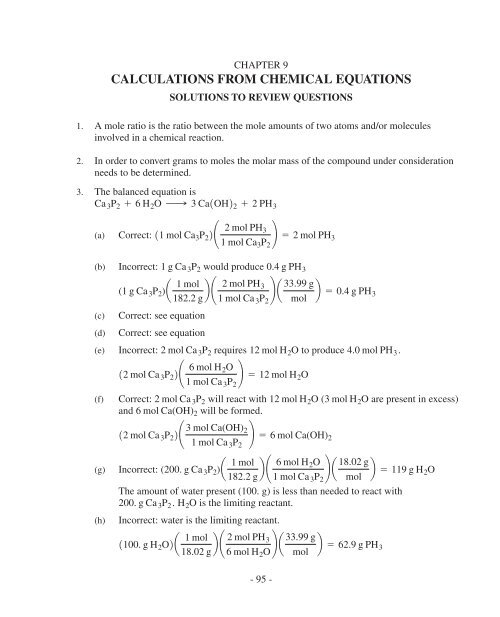
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 95<br />
CHAPTER 9<br />
CALCULATIONS FROM CHEMICAL EQUATIONS<br />
SOLUTIONS TO REVIEW QUESTIONS<br />
1. A mole ratio is the ratio between the mole amounts of two atoms and/or molecules<br />
involved in a chemical reaction.<br />
2. In order to convert grams to moles the molar mass of the compound under consideration<br />
needs to be determined.<br />
3. The balanced equation is<br />
Ca 3 P 2 + 6 H 2 O ¡ 3 Ca1OH2 2 + 2 PH 3<br />
(a)<br />
Correct:<br />
11 mol Ca 3 P 2 2¢ 2 mol PH 3<br />
1 mol Ca 3 P 2<br />
≤ = 2 mol PH 3<br />
(b) Incorrect: 1 g Ca 3 P 2 would produce 0.4 g PH 3<br />
(c)<br />
(d)<br />
(1 g Ca 3 P 2 )a 1 mol<br />
182.2 g b ¢ 2 mol PH 3<br />
≤ a 33.99 g b = 0.4 g PH<br />
1 mol Ca 3 P 2 mol<br />
3<br />
Correct: see equation<br />
Correct: see equation<br />
(e) Incorrect: 2 mol Ca 3 P 2 requires 12 mol H 2 O to produce 4.0 mol PH 3 .<br />
12 mol Ca 3 P 2 2¢ 6 mol H 2O<br />
1 mol Ca 3 P 2<br />
≤ = 12 mol H 2 O<br />
(f) Correct: 2 mol Ca 3 P 2 will react with 12 mol H 2 O ( 3 mol H 2 O are present in excess)<br />
and 6 mol Ca(OH) 2 will be formed.<br />
12 mol Ca 3 P 2 2¢ 3 mol Ca(OH) 2<br />
1 mol Ca 3 P 2<br />
≤ = 6 mol Ca(OH) 2<br />
(g)<br />
(h)<br />
Incorrect: (200. g Ca 3 P 2 )a 1 mol<br />
182.2 g b ¢ 6 mol H 2O<br />
≤ a 18.02 g b = 119 g H<br />
1 mol Ca 3 P 2 mol<br />
2 O<br />
The amount of water present (100. g) is less than needed to react with<br />
200. g Ca 3 P 2 . H 2 O is the limiting reactant.<br />
Incorrect: water is the limiting reactant.<br />
1100. g H 2 O2a 1 mol<br />
18.02 g b ¢ 2 mol PH 3<br />
6 mol H 2 O ≤ a 33.99 g b = 62.9 g PH<br />
mol<br />
3<br />
- 95 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 96<br />
- <strong>Chapter</strong> 9 -<br />
4. The balanced equation is<br />
2 CH 4 + 3 O 2 + 2 NH 3 ¡ 2 HCN + 6 H 2 O<br />
(a)<br />
Correct<br />
2 mol HCN<br />
(b) Incorrect: 116 mol O 2 2¢ ≤ = 10.7 mol HCN (not 12 mol HCN)<br />
3 mol O 2<br />
(c)<br />
Correct<br />
(d) Incorrect: 112 mol HCN2¢ 6 mol H 2O<br />
(not 4 mol H 2 O)<br />
2 mol HCN ≤ = 36 mol H 2O<br />
(e) Correct<br />
O 2<br />
(f) Incorrect: is the limiting reactant<br />
2 mol HCN<br />
13 mol O 2 2¢ ≤ = 2 mol HCN<br />
3 mol O 2<br />
(not 3 mol HCN)<br />
5. The theoretical yield of a chemical reaction is the maximum amount of product that can<br />
be produced based on a balanced equation. The actual yield of a reaction is the actual<br />
amount of product obtained.<br />
6. You can calculate the percent yield of a chemical reaction by dividing the actual yield by<br />
the theoretical yield and multiplying by one hundred.<br />
- 96 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 97<br />
CHAPTER 9<br />
SOLUTIONS TO EXERCISES<br />
1. (a)<br />
(b)<br />
(c)<br />
(d)<br />
2. (a)<br />
(b)<br />
(c)<br />
(d)<br />
3. (a)<br />
(b)<br />
(c)<br />
(d)<br />
(e)<br />
125.0 g KNO 3 2a 1 mol<br />
101.1 g b = 0.247 mol KNO 3<br />
1 mol<br />
156 mmol NaOH2a b = 0.056 mol NaOH<br />
1000 mmol<br />
15.4 * 10 2 g (NH 4 ) 2 C 2 O 4 2a 1 mol<br />
124.1 g b = 4.4 mol (NH 4) 2 C 2 O 4<br />
The conversion is: mL sol ¡ g sol ¡ g H 2 SO 4 ¡ mol H 2 SO 4<br />
116.8 mL solution2a 1.727 g<br />
mL b ¢ 0.800 g H 2SO 4<br />
≤ a 1 mol<br />
g solution 98.09 g b = 0.237 mol H 2SO 4<br />
12.10 kg NaHCO 3 2a 1000 g ba 1 mol<br />
kg 84.01 g b = 25.0 mol NaHCO 3<br />
1 g 1 mol<br />
1525 mg ZnCl 2 2a ba<br />
1000 mg 136.3 g b = 3.85 * 10-3 mol ZnCl 2<br />
19.8 * 10 24 1 mol<br />
molecules CO 2 2¢<br />
6.022 * 10 23 molecules ≤ = 16 mol CO 2<br />
1250 mL C 2 H 5 OH2a 0.789 g<br />
mL<br />
12.55 mol Fe(OH) 3 2a 106.9 g b = 273 g Fe(OH) 3<br />
mol<br />
1125 kg CaCO 3 2a 1000 g b = 1.25 * 10 5 g CaCO<br />
kg<br />
3<br />
110.5 mol NH 3 2a 17.03 g b = 179 g NH<br />
mol<br />
3<br />
1 mol<br />
ba<br />
46.07 g b = 4.3 mol C 2H 5 OH<br />
1 mol<br />
172 mmol HCl2a<br />
1000 mmol ba36.46 g b = 2.6 g HCl<br />
mol<br />
1500.0 mL Br 2 2a 3.119 g<br />
mL b = 1559.5 g Br 2 = 1.560 * 10 3 g Br 2<br />
- 97 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 98<br />
- <strong>Chapter</strong> 9 -<br />
4. (a)<br />
(b)<br />
(c)<br />
(d)<br />
(e)<br />
(0.00844 mol NiSO 4 )a 154.8 g b = 1.31 g NiSO<br />
mol<br />
4<br />
(0.0600 mol HC 2 H 3 O 2 )a 60.05 g b = 3.60 g HC 2 H 3 O 2<br />
mol<br />
(0.725 mol Bi 2 S 3 )a 514.2 g b = 373 g Bi<br />
mol<br />
2 S 3<br />
(4.50 * 10 21 1 mol<br />
molecules C 6 H 12 O 6 )¢<br />
6.022 * 10 23 molecules ≤ a 180.2 g b<br />
mol<br />
= 1.35g C 6 H 12 O 6<br />
(75 mL solution2a 1.175 g<br />
mL ba0.200 g K 2CrO 4<br />
b = 18 g K<br />
g solution<br />
2 CrO 4<br />
5. Larger number of molecules: 10.0 g H 2 O or 10.0 g H 2 O 2<br />
Water has a lower molar mass than hydrogen peroxide. 10.0 grams of water has a lower<br />
molar mass, contains more moles, and therefore more molecules than 10.0 g of H 2 O 2 .<br />
6. Larger number of molecules: 25.0 g HCl or 85 g C 6 H 12 O 6<br />
125.0 g HCl2a 1 mol<br />
36.46 g ba6.022 * 1023 molecules<br />
b = 4.13 * 10 23 molecules HCl<br />
mol<br />
185.0 g C 6 H 12 O 6 2a 1 mol<br />
180.2 g ba6.022 * 1023 molecules<br />
b<br />
mol<br />
HCl contains more molecules<br />
= 2.84 * 10 23 molecules C 6 H 12 O 6<br />
7. Mole ratios<br />
2 C 3 H 7 OH + 9 O 2 ¡ 6 CO 2 + 8 H 2 O<br />
(a)<br />
(b)<br />
(c)<br />
6 mol CO 2<br />
8 mol H 2 O<br />
(d)<br />
2 mol C 3 H 7 OH<br />
2 mol C 3 H 7 OH<br />
2 mol C 3 H 7 OH<br />
6 mol CO 2<br />
(e)<br />
9 mol O 2 8 mol H 2 O<br />
9 mol O 2<br />
8 mol H 2 O<br />
(f)<br />
6 mol CO 2 9 mol O 2<br />
- 98 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 99<br />
- <strong>Chapter</strong> 9 -<br />
8. Mole ratios<br />
3 CaCl 2 + 2 H 3 PO 4 ¡ Ca 3 (PO 4 ) 2 + 6 HCl<br />
(a)<br />
(b)<br />
(c)<br />
3 mol CaCl 2<br />
1 mol Ca 3 (PO 4 ) 2<br />
(d)<br />
1 mol Ca 3 (PO 4 ) 2<br />
2 mol H 3 PO 4<br />
6 mol HCl<br />
6 mol HCl<br />
(e)<br />
2 mol H 3 PO 4 1 mol Ca 3 (PO 4 ) 2<br />
3 mol CaCl 2<br />
2 mol H 3 PO 4<br />
(f)<br />
2 mol H 3 PO 4 6 mol HCl<br />
9.<br />
C 2 H 5 OH + 3 O 2 ¡ 2 CO 2 + 3 H 2 O<br />
2 mol CO 2<br />
17.75 mol C 2 H 5 OH2¢<br />
1 mol C 2 H 5 OH ≤ = 15.5 mol CO 2<br />
10. The balanced equation is 4 HCl + O 2 ¡ 2 Cl 2 + 2 H 2 O<br />
15.60 mol HCl2¢ 2 mol Cl 2<br />
4 mol HCl ≤ = 2.80 mol Cl 2<br />
11. The balanced equation is<br />
MnO 2 (s) + 4 HCl(aq) ¡ Cl 2 (g) + MnCl 2 (aq) + 2 H 2 O(l)<br />
(a)<br />
(b)<br />
4 mol HCl<br />
11.05 mol MnO 2 2¢ ≤ = 4.20 mol HCl<br />
1 mol MnO 2<br />
11.25 mol H 2 O2¢ 1 mol MnCl 2<br />
2 mol H 2 O ≤ = 0.625 mol MnCl 2s<br />
12.<br />
Al 4 C 3 + 12 H 2 O ¡ 4 Al(OH) 3 + 3 CH 4<br />
(a)<br />
1100. g Al 4 C 3 2a 1 mol<br />
144.0 g b ¢ 12 mol H 2O<br />
1 mol Al 4 C 3<br />
≤ = 8.33 mol H 2 O<br />
(b) 10.600 mol CH 4 2¢ 4 mol Al(OH) 3<br />
3 mol CH 4<br />
≤ = 0.800 mol Al(OH) 3<br />
- 99 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 100<br />
- <strong>Chapter</strong> 9 -<br />
13. Grams of NaOH<br />
Ca(OH) 2 + Na 2 CO 3 ¡ 2 NaOH + CaCO 3<br />
The conversion is: g Ca(OH) 2 ¡ mol Ca(OH) 2 ¡ mol NaOH ¡ g NaOH<br />
1500 g Ca(OH) 2 2a 1 mol 2 mol NaOH<br />
ba ba 40.00 g b = 5 * 10 2 g NaOH<br />
74.10 g 1 mol Ca(OH) 2 mol<br />
14. Grams of Zn 3 (PO 4 ) 2<br />
3 Zn + 2 H 3 PO 4 ¡ Zn 3 (PO 4 ) 2 + 3 H 2<br />
The conversion is: g Zn ¡ mol Zn ¡ mol Zn 3 (PO 4 ) 2 ¡ g Zn 3 (PO 4 ) 2<br />
110.0 g Zn2a 1 mol<br />
65.39 g b ¢ 1 mol Zn 3(PO 4 ) 2<br />
≤ a 386.1 g b = 19.7 g Zn<br />
3 mol Zn mol<br />
3 (PO 4 ) 2<br />
15. The balanced equation is Fe 2 O 3 + 3 C ¡ 2 Fe + 3 CO<br />
The conversion is: kg Fe 2 O 3 ¡ kmol Fe 2 O 3 ¡ kmol Fe ¡ kg Fe<br />
1125 kg Fe 2 O 3 2a 1 kmol 2 kmol Fe 55.85 kg<br />
ba ba b = 87.4 kg Fe<br />
159.7 kg 1 kmol Fe 2 O 3 kmol<br />
16. The balanced equation is 3 Fe + 4 H 2 O ¡ Fe 3 O 4 + 4 H 2<br />
Calculate the grams of both H 2 O and Fe to produce 375 g Fe 3 O 4<br />
1375 g Fe 3 O 4 2¢ 1 mol<br />
231.6 g ≤ a 4 mol H 2O<br />
≤ a 18.02 g b = 117 g H<br />
1 mol Fe 3 O 4 mol<br />
2 O<br />
1375 g Fe 3 O 4 2a 1 mol 3 mol Fe<br />
b ¢ ≤ a 55.85 g b = 271 g Fe<br />
231.6 g 1 mol Fe 3 O 4 mol<br />
17. The balanced equation is 2 C 2 H 6 + 7 O 2 ¡ 4 CO 2 + 6 H 2 O<br />
(a)<br />
(b)<br />
(c)<br />
115.0 mol C 2 H 6 2¢ 7 mol O 2<br />
2 mol C 2 H 6<br />
≤ = 52.5 mol O 2<br />
18.00 g H 2 O2a 1 mol<br />
18.02 g b ¢ 4 mol CO 2<br />
6 mol H 2 O ≤ a 44.01 g b = 13.0 g CO<br />
mol<br />
2<br />
175.0 g C 2 H 6 2a 1 mol<br />
30.07 g b ¢ 4 mol CO 2<br />
≤ a 44.01 g b = 2.20 * 10 2 g CO<br />
2 mol C 2 H 6 mol<br />
2<br />
(d) 12.75 mol of H 2 O2¢ 4 mol CO 2<br />
6 mol H 2 O ≤ a 44.01 g b = 80.7 g CO<br />
mol<br />
2<br />
- 100 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 101<br />
- <strong>Chapter</strong> 9 -<br />
(e)<br />
125.0 mol C 2 H 6 2¢ 7 mol O 2<br />
2 mol C 2 H 6<br />
≤ a 32.00 g<br />
mol O 2<br />
b = 2.80 * 10 3 g O 2<br />
(f)<br />
1125 g H 2 O2¢ 1 mol<br />
18.02 g ≤¢2 mol C 2H 6<br />
6 mol H 2 O ≤ a 30.07 g b = 69.5 g C<br />
mol<br />
2 H 6<br />
18.<br />
4 FeS 2 + 11 O 2 ¡ 2 Fe 2 O 3 + 8 SO 2<br />
(a)<br />
11.00 mol FeS 2 2a 2 mol Fe 2O 3<br />
4 mol FeS 2<br />
b = 0.500 mol Fe 2 O 3<br />
(b)<br />
14.50 mol FeS 2 2¢ 11 mol O 2<br />
4 mol FeS 2<br />
≤ = 12.4 mol O 2<br />
(c)<br />
11.55 mol Fe 2 O 3 2¢ 8 mol SO 2<br />
2 mol Fe 2 O 3<br />
≤ = 6.20 mol SO 2<br />
(d)<br />
10.512 mol FeS 2 2¢ 8 mol SO 2<br />
≤ a 64.07 g b = 65.6 g SO<br />
4 mol FeS 2 mol<br />
2<br />
(e)<br />
140.6 g SO 2 2a 1 mol<br />
64.07 g b ¢ 11 mol O 2<br />
8 mol SO 2<br />
≤ = 0.871 mol O 2<br />
(f)<br />
1221 g Fe 2 O 3 2a 1 mol<br />
159.7 g b ¢ 4 mol FeS 2<br />
≤ a 120.0 g b = 332 g FeS<br />
2 mol Fe 2 O 3 mol<br />
2<br />
19. (a) Hydrogen Oxygen<br />
Hydrogen is the limiting reactant.<br />
- 101 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 102<br />
- <strong>Chapter</strong> 9 -<br />
(b)<br />
Hydrogen Bromine<br />
Bromine is the limiting reactant.<br />
20.<br />
(a)<br />
Lithium Iodine<br />
No limiting reactant.<br />
(b)<br />
Silver Chlorine<br />
Silver is the limiting reactant.<br />
21.<br />
(a)<br />
Potassium Chlorine<br />
Potassium is the limiting reactant.<br />
- 102 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 103<br />
- <strong>Chapter</strong> 9 -<br />
(b)<br />
Aluminum Oxygen<br />
Oxygen is the limiting reactant.<br />
22.<br />
(a)<br />
Nitrogen Oxygen<br />
Oxygen is the limiting reactant.<br />
(b)<br />
Iron Hydrogen Oxygen<br />
Water is the limiting reactant.<br />
23. (a)<br />
KOH + HNO 3 ¡ KNO 3 + H 2 O<br />
16.0 g 12.0 g<br />
Choose one of the products and calculate its mass that would be produced from<br />
each given reactant. Using KNO 3 as the product:<br />
116.0 g KOH2¢ 1 mol<br />
56.10 g ≤ a 1 mol KNO 3<br />
1 mol KOH ≤ a 101.1 g b = 28.8 g KNO<br />
mol<br />
3<br />
112.0 g HNO 3 2a 1 mol<br />
63.02 g b ¢ 1 mol KNO 3<br />
1 mol KOH ≤ a 101.1 g b = 19.3 g KNO<br />
mol<br />
3<br />
Since HNO 3 produces less KNO 3 , it is the limiting reactant and KOH is in excess.<br />
- 103 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 104<br />
- <strong>Chapter</strong> 9 -<br />
(b)<br />
2 NaOH + H 2 SO 4 ¡ Na 2 SO 4 + 2 H 2 O<br />
10.0 g 10.0 g<br />
Choose one of the products and calculate its mass that would be produced from<br />
each given reactant. Using H 2 O as the product:<br />
Since H 2 SO 4 produces less H 2 O, it is the limiting reactant and NaOH is in excess.<br />
24. (a) 2 Bi(NO 3 ) 3 + 3 H 2 S ¡ Bi 2 S 3 + 6 HNO 3<br />
50.0 g 6.00 g<br />
Choose one of the products and calculate its mass that would be produced from<br />
each given reactant. Using Bi 2 S 3 as the product:<br />
(b)<br />
110.0 g NaOH2a 1 mol<br />
40.00 g b ¢ 2 mol H 2O<br />
2 mol NaOH ≤ a 18.02 g b = 4.51 g H<br />
mol<br />
2 O<br />
110.0 g H 2 SO 4 2a 1 mol<br />
98.09 g b ¢ 2 mol H 2O<br />
≤ a 18.02 g b = 3.67 g H<br />
1 mol H 2 SO 4 mol<br />
2 O<br />
150.0 g Bi(NO 3 ) 3 )a 1 mol<br />
395.0 g ba 1 mol Bi 2S 3<br />
ba 514.2 g b = 32.5 g Bi<br />
2 mol Bi(NO 3 ) 3 mol<br />
2 S 3<br />
(6.00 g H 2 S)a 1 mol<br />
34.09 g b ¢ 1 mol Bi 2S 3<br />
3 mol H 2 S ≤ a 514.2 g b = 30.2 g Bi<br />
mol<br />
2 S 3<br />
Since H 2 S produces less Bi 2 S 3 , it is the limiting reactant and Bi(NO 3 ) 3 is in excess.<br />
3 Fe + 4 H 2 O ¡ Fe 3 O 4 + 4 H 2<br />
40.0 g 16.0 g<br />
Choose one of the products and calculate its mass that would be produced from<br />
each given reactant. Using H 2 as the product:<br />
(40.0 g Fe)a 1 mol<br />
55.85 g b ¢ 4 mol H 2<br />
3 mol Fe ≤ a 2.016 g b = 1.93 g H<br />
mol<br />
2<br />
116.0 g H 2 O2a 1 mol<br />
18.02 g b ¢ 4 mol H 2<br />
4 mol H 2 O ≤ a 2.016 g b = 1.79 g H<br />
mol<br />
2<br />
Since H 2 O produces less H 2 , it is the limiting reactant and Fe is in excess.<br />
25. Limiting reactant calculations<br />
C 3 H 8 + 5 O 2 ¡ 3 CO 2 + 4 H 2 O<br />
(a) Reaction between 20.0 g C 3 H 8 and 20.0 g O 2<br />
Convert each amount to moles of CO 2<br />
- 104 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 105<br />
- <strong>Chapter</strong> 9 -<br />
120.0 g C 3 H 8 2a 1 mol<br />
44.09 g b ¢ 3 mol CO 2<br />
1 mol C 3 H 8<br />
≤ = 1.36 moles CO 2<br />
120.0 g O 2 2a 1 mol<br />
32.00 g b ¢ 3 mol CO 2<br />
5 mol O 2<br />
≤ = 0.375 moles CO 2<br />
O 2 is the limiting reactant. The yield is 0.375 moles CO 2 .<br />
(b) Reaction between 20.0 g C 3 H 8 and 80.0 g O 2<br />
Convert each amount to moles of CO 2<br />
120.0 g C 3 H 8 2a 1 mol<br />
44.09 g b ¢ 3 mol CO 2<br />
1 mol C 3 H 8<br />
≤ = 1.36 moles CO 2<br />
180.0 g O 2 2a 1 mol<br />
32.00 g b ¢ 3 mol CO 2<br />
5 mol O 2<br />
≤ = 1.50 moles CO 2<br />
C 3 H 8 is the limiting reactant. The yield is 1.36 moles CO 2 .<br />
(c) Reaction between 2.0 mol C 3 H 8 and 14.0 mol O 2<br />
According to the equation, 2 mol C 3 H 8 will react with 10 mol O 2 . Therefore, C 3 H 8<br />
is the limiting reactant and 4.0 mol O 2 will remain unreacted.<br />
12.0 mol C 3 H 8 2¢ 3 mol CO 2<br />
1 mol C 3 H 8<br />
≤ = 6.0 mol CO 2 produced<br />
(2.0 mol C 3 H 8 )¢ 4 mol H 2O<br />
1 mol C 3 H 8<br />
≤ = 8.0 mol H 2 O produced<br />
When the reaction is completed, 6.0 mol CO 2 , 8.0 H 2 O, and 4.0 mol O 2 will be in<br />
the container.<br />
26. The balanced equation is 2 C 3 H 6 + 9 O 2 ¡ 6 CO 2 + 6 H 2 O<br />
(a) Reaction between 15.0 g C 3 H 6 and 15.0 g O 2 .<br />
Convert each amount to moles of H 2 O<br />
115.0 g C 3 H 6 2¢ 1 mol<br />
42.08 g ≤¢6 mol H 2O<br />
2 mol C 3 H 6<br />
≤ = 1.07 mol H 2 O<br />
115.0 g O 2 2¢ 1 mol<br />
32.00 g ≤¢6 mol H 2O<br />
2 mol O 2 ≤ = 0.313 mol H 2O<br />
The O 2 is the limiting reactant. The yield is 0.313 mol H 2 O.<br />
- 105 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 106<br />
- <strong>Chapter</strong> 9 -<br />
(b)<br />
Reaction between 12.0 g of C 3 H 6 and 25.0 g of O 2 .<br />
Convert each amount to moles of H 2 O<br />
112.0 g C 3 H 6 2¢ 1 mol<br />
42.08 g ≤¢6 mol H 2O<br />
2 mol C 3 H 6<br />
≤ = 0.856 mol H 2 O<br />
125.0 g O 2 2¢ 1 mol<br />
32.00 g ≤¢6 mol H 2O<br />
9 mol O 2<br />
≤ = 0.521 mol H 2 O<br />
O 2<br />
is the limiting reactant. The yield is 0.521 mol H 2 O.<br />
(c) Reaction between 5.0 mol of C 3 H 6 and 15.0 mol of O 2 .<br />
Convert each to moles of CO 2<br />
(5.0 mol C 3 H 6 )¢ 6 mol CO 2<br />
2 mol C 3 H 6<br />
≤ = 15 mol CO 2<br />
(15.0 mol O 2 )¢ 6 mol CO 2<br />
≤ = 10 mol CO<br />
9 mol O 2<br />
2<br />
Since is the limiting reactant. C 3 H 6 will be left unreacted.<br />
O 2<br />
27.<br />
28.<br />
X 8 + 12 O 2 ¡ 8 XO 3<br />
The conversion is: g O 2 ¡ mol O 2 ¡ mol X 8<br />
(120.0 g O 2 2a 1 mol<br />
32.00 g b ¢ 1 mol X 8<br />
12 mol O 2<br />
≤ = 0.3125 mol X 8 80.0 g X 8 = 0.3125 mol X 8<br />
80.0 g<br />
0.3125 mol = 256 g>mol X 8<br />
g<br />
256<br />
mol g<br />
molar mass X = = 32.0<br />
8 mol<br />
Using the periodic table we find that the element with 32.0 g>mol is sulfur.<br />
X + 2 HCl ¡ XCl 2 + H 2<br />
The conversion is: g H 2 ¡ mol H 2 ¡ mol X<br />
12.42 g H 2 2a 1 mol<br />
2.016 g b ¢ 1 mol X<br />
1 mol H 2<br />
≤ = 1.20 mol X 78.5 g X = 1.20 molX<br />
78.5 g<br />
= 65.4 g>mol<br />
1.20 mol<br />
Using the periodic table we find that the element with atomic mass 65.4 is zinc.<br />
- 106 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 107<br />
- <strong>Chapter</strong> 9 -<br />
29. Limiting reactant calculation and percentage yield<br />
2 Al + 3 Br 2 ¡ 2 AlBr 3<br />
Reaction between 25.0 g Al and 100. g Br 2<br />
Calculate the grams of AlBr 3 from each reactant.<br />
125.0 g Al2a 1 mol<br />
26.98 g b ¢ 2 mol AlBr 3<br />
≤ a 266.7 g b = 247 g AlBr<br />
2 mol Al mol<br />
3<br />
(100. g Br 2 )a 1 mol<br />
159.8 g b ¢ 2 mol AlBr 3<br />
≤ a 266.7 g b = 111 g AlBr<br />
3 mol Br 2 mol<br />
3<br />
Br 2 is limiting; 111 g AlBr 3 is the theoretical yield of product.<br />
actual yield<br />
64.2 g<br />
Percent yield = a<br />
b11002 = a b11002 = 57.8%<br />
theoretical yield 111 g<br />
30. Percent yield calculation<br />
Fe(s) + CuSO 4 (aq) ¡ Cu(s) + FeSO 4 (aq)<br />
1400. g CuSO 4 2a 1 mol 1 mol Cu<br />
b ¢ ≤ a 63.55 g b = 159 g Cu (theoretical yield)<br />
159.6 g 1 mol CuSO 4 mol<br />
actual yield<br />
151 g<br />
% yield = a<br />
b11002 = a b11002 = 95.0% yield of Cu<br />
theoretical yield 159 g<br />
31. The balanced equation is 3 C + 2 SO 2 ¡ CS 2 + 2 CO 2<br />
Calculate the g C needed to produce 950 g CS 2 taking into account that the yield of CS 2<br />
is 86.0%. First calculate the theoretical yield of CS 2 .<br />
950 g CS 2<br />
0.860<br />
= 1.1 * 10 3 g CS 2 1theoretical yield2<br />
Now calculate the grams of coke needed to produce 1.1 * 10 3 g CS 2 .<br />
11.1 * 10 3 g CS 2 2a 1 mol<br />
76.15 g b ¢ 3 mol C ≤ a 12.01 g b = 5.2 * 10 2 g C<br />
1 mol CS 2 mol<br />
32. The balanced equation is CaC 2 + 2 H 2 O ¡ C 2 H 2 + Ca1OH2 2<br />
First calculate the grams of pure CaC 2 in the sample from the amount of C 2 H 2 produced.<br />
10.540 mol C 2 H 2 2¢ 1 mol CaC 2<br />
≤ a 64.10 g CaC 2<br />
b = 34.6 g of pure CaC<br />
1 mol C 2 H 2 mol CaC 2 in the sample<br />
2<br />
- 107 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 108<br />
- <strong>Chapter</strong> 9 -<br />
Now calculate the percent<br />
in the impure sample.<br />
33. No. There are not enough screwdrivers, wrenches or pliers. 2400 screwdrivers,<br />
3600 wrenches and 1200 pliers are needed for 600 tool sets.<br />
34. A subscript is used to indicate the number of atoms in a formula. It cannot be changed<br />
without changing the identity of the substance. Coefficients are used only to balance atoms in<br />
chemical equations. They may be changed as needed to achieve a balanced equation.<br />
35. Consider the reaction A ¡ 2B and assume that you have 1 gram of A. This does not<br />
guarantee that you will produce 1 gram of B because A and B have different molar<br />
masses. One gram of A does not contain the same number of molecules as 1 gram of B.<br />
However, 1 mole of A does have the same number of molecules as one mole of B.<br />
(Remember, 1 mole = 6.022 * 10 23 molecules always.) If you determine the number of<br />
moles in one gram of A and multiply by 2 to get the number of moles of B then from<br />
that you can determine the grams of B using its molar mass. Equations are written in<br />
terms of moles not grams.<br />
36.<br />
(a)<br />
CaC 2<br />
- 108 -<br />
¢ 34.6 g CaC 2<br />
44.5 g sample ≤11002 = 77.8% CaC 2 in the impure sample<br />
4 KO 2 + 2 H 2 O + 4 CO 2 ¡ 4 KHCO 3 + 3 O 2<br />
Á<br />
¢ 0.85 g CO 2<br />
≤ a 1 mol<br />
min 44.01 g b ¢ 4 mol KO 2<br />
≤ = 0.019 mol KO 2<br />
4 mol CO 2 min<br />
¢ 0.019 mol KO 2<br />
b110.0 min2 = 0.19 mol KO<br />
min<br />
2<br />
(b)<br />
37. (a)<br />
The conversion is:<br />
g CO 2<br />
min ¡ mol CO 2<br />
min<br />
¡ mol O 2<br />
min<br />
¢ 0.85 g CO 2<br />
≤ a 1 mol<br />
min 44.01 g b ¢ 3 mol O 2<br />
≤ a 32.00 g<br />
4 mol CO 2 mol<br />
1750 g C 6 H 12 O 6 2a 1 mol<br />
180.2 g b ¢ 2 mol C 2H 5 OH<br />
≤ a 46.07 g b = 380 g C<br />
1 mol C 6 H 12 O 6 mol<br />
2 H 5 OH<br />
ba<br />
¡ g O 2<br />
min ¡ g O 2<br />
hr<br />
60.0 min<br />
b = 28 g O 2<br />
1.0 hr hr<br />
1750 g C 6 H 12 O 6 2a 1 mol<br />
180.2 g b ¢ 2 mol CO 2<br />
≤ a 44.01 g b = 370 g CO<br />
1 mol C 6 H 12 O 6 mol<br />
2<br />
Alternate Solution: 750 g C 2 H 6 O 6 - 380 g<br />
C 2 H 5 OH = 370 g CO 2 by the conservation of mass method.<br />
(b)<br />
1380 g C 2 H 5 OH2a 1 mL<br />
0.79 g b = 480 mL C 2H 5 OH
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 109<br />
- <strong>Chapter</strong> 9 -<br />
38.<br />
2 CH 3 OH + 3 O 2 ¡ 2 CO 2 + 4 H 2 O<br />
The conversion is:<br />
mL CH 3 OH ¡ g CH 3 OH ¡ mol CH 3 OH ¡ mol O 2 ¡ g O 2<br />
160.0 mL CH 3 OH2a 0.72 g<br />
mL b ¢ 1 mol<br />
32.04 g ≤¢ 3 mol O 2<br />
2 mol CH 3 OH ≤ a 32.00 g b = 65 g O<br />
mol<br />
2<br />
39. The balanced equation is<br />
The conversion is:<br />
7 H 2 O 2 + N 2 H 4 ¡ 2 HNO 3 + 8 H 2 O<br />
(a)<br />
175 kg N 2 H 4 2a 1000 g<br />
1 kg b ¢ 1 mol<br />
32.05 g ≤¢2 mol HNO 3<br />
≤ a 63.02 g b = 2.9 * 10 5 g HNO<br />
1 mol N 2 H 4 mol<br />
3<br />
(b)<br />
(c)<br />
1250 L H 2 O 2 2a<br />
1000 mL<br />
1 L<br />
b ¢ 1.41 g 1 mol<br />
≤¢<br />
1 mL 34.02 g ≤ a 8 mol H 2O<br />
ba 18.02 g<br />
7 mol H 2 O 2 mol<br />
1725 g H 2 O 2 2a 1 mol<br />
34.02 g b ¢ 1 mol N 2H 4<br />
≤ a 32.05 g b = 97.6 g N<br />
7 mol H 2 O 2 mol<br />
2 H 4<br />
b<br />
= 2.1 * 10 5 g H 2 O<br />
(d) Reaction between 750 g of N 2 H 2 and 125 g of H 2 O 2 .<br />
Convert each amount to grams of H 2 O.<br />
1750 g N 2 H 4 2a 1 mol<br />
32.05 g b ¢ 8 mol H 2O<br />
≤ a 18.02 g b = 3.4 * 10 3 g H<br />
1 mol N 2 H 4 mol<br />
2 O<br />
1125 g H 2 O 2 2a 1 mol<br />
34.02 g b ¢ 8 mol H 2O<br />
≤ a 18.02 g b = 75.7 g H<br />
7 mol H 2 O 2 mol<br />
2 O<br />
75.7 g H 2 O can be produced.<br />
(e) Since H 2 O 2 is the limiting reactant, N 2 H 4 is in excess.<br />
1125 g H 2 O 2 2a 1 mol<br />
34.02 g b ¢ 1 mol N 2H 4<br />
≤ a 32.05 g b = 16.8 g N<br />
7 mol H 2 O 2 mol<br />
2 H 4 reacted<br />
750 g N 2 H 4 given - 16.8 g N 2 H 4 used = 730 g N 2 H 4 remaining<br />
- 109 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 110<br />
- <strong>Chapter</strong> 9 -<br />
40. The balanced equation is<br />
16 HCl + 2 KMnO 4 ¡ 5 Cl 2 + 2 KCl + 2 MnCl 2 + 8 H 2 O<br />
(a) Reaction between 25 g KMnO 4 and 85 g HCl. Convert each to moles of MnCl 2 .<br />
(b)<br />
125 g KMnO 4 2a 1 mol KMnO 4<br />
158.04 g KMnO 4<br />
ba 2 mol MnCl 2<br />
2 mol KMnO 4<br />
b = 0.16 mol MnCl 2<br />
185 g HCl2a 1 mol<br />
36.46 g ba2 mol MnCl 2<br />
16 mol HCl b = 0.29 mol MnCl 2<br />
KMnO 4 is the limiting reactant; 0.16 mol MnCl 2 produced.<br />
175 g KCl2a 1 mol<br />
74.55 g ba8 mol H 2O<br />
2 mol KCl ba18.02 g b = 73 g H<br />
mol<br />
2 O<br />
(c)<br />
Theoretical yield is 91 g Cl 2 ; Percent yield: a 75 g b(100) = 82% yield<br />
91 g<br />
(d) Reaction between 25 g HCl and 25 g KMnO 4 . Convert each amount to grams of CL 2 .<br />
(e)<br />
1150 g HCl2a 1 mol<br />
36.46 g ba 5 mol Cl 2<br />
16 mol HCl ba70.90 g b = 91 g Cl<br />
mol<br />
2<br />
125 g HCl2a 1 mol<br />
36.46 g ba 5 mol Cl 2<br />
16 mol HCl ba70.90 g b = 15 g Cl<br />
mol<br />
2<br />
125 g KMnO 4 2a 1 mol<br />
158.04 g ba 5 mol Cl 2<br />
ba 70.90 g b = 28 g Cl<br />
2 mol KMnO 4 mol<br />
2<br />
HCl is the limiting; KMnO 4 is in excess; 15 g Cl 2 will be produced.<br />
Calculate the mass of unreacted KMnO 4 :<br />
125 g HCl2a 1 mol<br />
.<br />
36.46 g ba2 mol KMnO 4<br />
ba 158.04 g b = 14 g KMnO<br />
16 mol HCl mol<br />
4 will react<br />
Unreacted KMnO 4 = 25 g -14 g = 11 g KMnO 4 remain unreacted.<br />
41. The balanced equation is<br />
4Ag + 2 H 2 S + O 2 ¡ 2 Ag 2 S + 2 H 2 O<br />
(a)<br />
11.1 g Ag2a 1 mol<br />
107.9 g ba2 mol Ag 2S<br />
4 mol Ag ba247.9 g b = 1.3 g Ag<br />
mol<br />
2 S<br />
10.14 g H 2 S2a 1 mol<br />
34.09 g ba2 mol Ag 2S<br />
2 mol H 2 S ba247.9 g b = 1.0 g Ag<br />
mol<br />
2 S<br />
- 110 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 111<br />
- <strong>Chapter</strong> 9 -<br />
(b)<br />
H 2 S is limiting 1.0 g Ag 2 S forms.<br />
0.17 g - 0.14 g = 0.03 grams more H 2 S needed to completely react Ag.<br />
42. Mass of the beaker<br />
26.500 g beaker + Ca(OH) 2<br />
26.095 g beaker + CaO<br />
0.405 g H 2 O absorbed<br />
10.405 g H 2 O2a 1 mol<br />
18.02 g b = 2.25 * 10-2 mol H 2 O absorbed<br />
Since the reaction is a 1:1 mole, the amount of CaO in the beaker is 2.25 * 10 -2 mol.<br />
Convert to grams.<br />
12.25 * 10 -2 56.08 g CaO<br />
mol CaO2a b = 1.26 g CaO in the beaker.<br />
26.095 g<br />
-1.26 g<br />
24.835 g<br />
10.080 g O 2 2a 1 mol<br />
32.00 g ba2 mol Ag 2S<br />
ba 247.9 g b = 1.2 g Ag<br />
1 mol O 2 mol<br />
2 S<br />
11.1 g Ag2a 1 mol<br />
107.9 g ba2 mol H 2S<br />
4 mol Ag ba34.09 g b = 0.17 g H<br />
mol<br />
2 S reacts<br />
mol<br />
beaker + CaO<br />
CaO<br />
mass of the beaker<br />
43.<br />
Pb(NO 3 ) 2 (aq) + 2 KI(aq) ¡ PbI 2 (s) + 2 KNO 3 (aq)<br />
(a) The solid is lead (II) iodide, PbI 2 .<br />
(b) Double displacement reaction.<br />
(c) Calculate the moles of each reactant.<br />
[15 g Pb(NO 3 ) 2 ] + a 1 mol<br />
331.2 g b = 0.045 mol Pb(NO 3) 2<br />
115 g KI2a 1 mol b = 0.090 mol KI<br />
166.0 g<br />
Stoichiometric quantities of reactants are used.<br />
Theoretical yield of PbI 2 is 0.045 mol.<br />
Actual yield:<br />
Percent yield: a<br />
16.68 g PbI 2 2a 1 mol<br />
461.1 g b = 0.0145 mol PbI 2<br />
0.0145 mol<br />
b11002 = 32% yield<br />
0.045 mol<br />
- 111 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 112<br />
- <strong>Chapter</strong> 9 -<br />
44. Composition of a mixture of and KCl.<br />
In the mixture only KCl reacts with AgNO 3 .<br />
KCl(aq) + AgNO 3 (aq) ¡ AgCl(s) + KNO 3 (aq)<br />
74.55 g KCl<br />
14.33 g AgCl2a b = 2.25 g KCl in the mixture<br />
143.7 g AgCl<br />
KNO 3<br />
- 112 -<br />
10.00 g mixture - 2.25 g KCl = 7.75 g KNO 3<br />
2.25 g KCl<br />
a b11002 = 22.5% KCl<br />
10.00 g mixture<br />
a 7.75 g KNO 3<br />
10.00 g mixture b11002 = 77.5% KNO 3<br />
45. The balanced equation is Zn + 2 HCl ¡ ZnCl 2 + H 2<br />
180.0 g Zn - 35 g Zn = 145 g Zn reacted with HCl<br />
(a)<br />
(b)<br />
(c)<br />
1145 g Zn2a 1 mol<br />
65.39 g ba1 mol H 2<br />
1 mol Zn b a 2.016 g b = 4.47 g H<br />
mol<br />
2 produced<br />
1145 g Zn2a 1 mol mol HCl<br />
ba2<br />
65.39 g 1 mol Zn ba36.46 g b = 162 g HCl reacted<br />
mol<br />
1180.0 g Zn2a 1 mol mol HCl<br />
ba2<br />
65.39 g 1 mol Zn ba36.46 g b = 201 g HCl reacts<br />
mol<br />
201 g - 162 g = 39 g more HCl needed to react wih the 180.0 g Zn<br />
46.<br />
Fe(s) + CuSO 4 (aq)<br />
2.0 mol 3.0 mol<br />
¡<br />
Cu(s)<br />
FeSO 4 (aq)<br />
(a) 2.0 mol Fe react with 2.0 mol CuSO 4 to yield 2.0 mol Cu and 2.0 mol FeSO 4 .<br />
1.0 mol CuSO 4 is unreacted. At the completion of the reaction, there will be<br />
2.0 mol Cu, 2.0 mol FeSO 4 , and 1.0 mol CuSO 4 .<br />
(b) Determine which reactant is limiting and then calculate the g FeSO 4 produced from<br />
that reactant.<br />
120.0 g Fe2a 1 mol mol Cu<br />
ba1<br />
55.85 g 1 mol Fe ba63.55 g b = 22.8 g Cu<br />
mol<br />
140.0 g CuSO 4 2a 1 mol 1 mol Cu<br />
ba ba 63.55 g b = 15.9 g Cu<br />
159.6 g 1 mol CuSO 4 mol<br />
Since CuSO 4 produces less Cu, it is the limiting reactant. Determine the mass of<br />
FeSO 4 produced from 40.0 g CuSO 4 .<br />
+
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 113<br />
- <strong>Chapter</strong> 9 -<br />
140.0 g CuSO 4 2a 1 mol<br />
159.6 g b ¢ 1 mol FeSO 4<br />
≤ a 151.9 g b = 38.1 g FeSO<br />
1 mol CuSO 4 mol<br />
4 produced<br />
Calculate the mass of unreacted Fe.<br />
140.0 g CuSO 4 2a 1 mol<br />
159.6 g b ¢ 1 mol Fe<br />
≤ a 55.85 g b = 14.0 g Fe will react<br />
1 mol CuSO 4 mol<br />
Unreacted Fe = 20.0 g - 14.0 g = 6.0 g. Therefore, at the completion of the<br />
reaction, 15.9 g Cu, 38.1 g FeSO 4 , 6.0 g Fe, and no CuSO 4 remain.<br />
47. Limiting reactant calculation<br />
CO(g) + 2 H 2 (g) ¡ CH 3 OH(l)<br />
Reaction between 40.0 g CO and 10.0 g H 2 : determine the limiting reactant by<br />
calculating the amount of CH 3 OH that would be formed from each reactant.<br />
140.0 g CO2a 1 mol<br />
28.01 g b ¢ 1 mol CH 3OH<br />
1 mol CO ≤ a 32.04 g b = 45.8 g CH<br />
mol<br />
3 OH<br />
110.0 g H 2 2a 1 mol<br />
2.016 g b ¢ 1 mol CH 3OH<br />
≤ a 32.04 g b = 79.5 g CH<br />
2 mol H 2 mol<br />
3 OH<br />
CO is limiting; H 2 is in excess; 45.8 g CH 3 OH will be produced.<br />
Calculate the mass of unreacted H 2 :<br />
140.0 g CO2a 1 mol<br />
28.01 g b ¢ 2 mol H 2<br />
1 mol CO ≤ a 2.016 g b = 5.76 g H<br />
mol<br />
2 react<br />
10.0 g H 2 - 5.76 g H 2 = 4.2 g H 2 remain unreacted<br />
48. The balanced equation is C 6 H 12 O 6 ¡ 2 C 2 H 5 OH + 2 CO 2<br />
(a) First calculate the theoretical yield.<br />
1750 g C 6 H 12 O 6 2a 1 mol<br />
180.2 g b ¢ 2 mol C 2H 5 OH<br />
≤ a 46.07 g b<br />
1 mol C 6 H 12 O 6 mol<br />
Then take 84.6% of the theoretical yield to obtain the actual yield.<br />
actual yield =<br />
1theoretical yield2184.62<br />
100<br />
= 3.2 * 10 2 g C 2 H 5 OH<br />
- 113 -<br />
= 3.8 * 10 2 g C 2 H 5 OH (theoretical yield)<br />
= 13.8 * 102 g C 2 H 5 OH2184.62<br />
100
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 114<br />
- <strong>Chapter</strong> 9 -<br />
(b) 475 g C 2 H 5 OH represents 84.6% of the theoretical yield. Calculate the<br />
theoretical yield.<br />
theoretical yield = 475 g<br />
0.846 = 561 g C 2H 5 OH<br />
Now calculate the g C 6 H 12 O 6 needed to produce 561 g C 2 H 5 OH.<br />
1561 g C 2 H 5 OH2a 1 mol<br />
46.07 g b ¢ 1 mol C 6H 12 O 6<br />
2 mol C 2 H 5 OH ≤ a 180.2 g b = 1.10 * 10 3 g C<br />
mol<br />
6 H 12 O 6<br />
49. The balanced equations are:<br />
CaCl 2 (aq) + 2 AgNO 3 (aq) ¡ Ca(NO 3 ) 2 (aq) + 2 AgCl(s)<br />
MgCl 2 (aq) + 2 AgNO 3 (aq) ¡ Mg1NO 3 2 2 (aq) + 2 AgCl(s)<br />
1 mol of each salt will produce the same amount (2 mol) of AgCl. MgCl 2 has a higher<br />
percentage of Cl than CaCl 2 because Mg has a lower atomic mass than Ca. Therefore, on<br />
an equal mass basis, MgCl 2 will produce more AgCl than will CaCl 2 .<br />
Calculations show that 1.00 g MgCl 2 produces 3.01 g AgCl, and 1.00 g CaCl 2 produces<br />
2.56 g AgCl.<br />
50. The balanced equation is Li 2 O + H 2 O ¡ 2 LiOH<br />
The conversion is: g H 2 O ¡ mol H 2 O ¡ mol Li 2 O ¡ g Li 2 O ¡ kg Li 2 O<br />
¢ 2500 g H 2O<br />
astronaut day ≤ a 1 mol<br />
18.02 g b ¢ 1 mol Li 2O<br />
¢ 4.1 kg Li 2O<br />
astronaut day ≤130 days213 astronauts2 = 3.7 * 102 kg Li 2 O<br />
51. The balanced equation is<br />
H 2 SO 4 + 2 NaCl ¡ Na 2 SO 4 + 2 HCl<br />
First calculate the g HCl to be produced<br />
120.0 L HCl solution2a<br />
Then calculate the g H 2 SO 4 required to produce the HCl<br />
11.01 * 10 4 g HCl2a 1 mol<br />
36.46 g b ¢ 1 mol H 2SO 4<br />
≤ a 98.09 g<br />
2 mol HCl 1 mol b = 1.36 * 104 g H 2 SO 4<br />
Finally, calculate the kg H 2 SO 4 (96%)<br />
1 mol H 2 O ≤ a 29.88 g<br />
mol<br />
ba 1 kg<br />
1000 g b = 4.1 kg Li 2O<br />
astronaut day<br />
1000 mL<br />
ba 1.20 g<br />
1 L 1.00 mL b10.4202 = 1.01 * 104 g HCl<br />
11.36 * 10 4 g H 2 SO 4 2¢ 1.00 g H 2SO 4 solution<br />
0.96 g H 2 SO 4<br />
≤ a 1 kg<br />
1000 g b = 14 kg concentrated H 2SO 4<br />
- 114 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 115<br />
- <strong>Chapter</strong> 9 -<br />
52. Percent yield of H 2 SO 4<br />
1100.0 g S2a 1 mol b = 3.118 mol S to start with<br />
32.07 g<br />
3.118 mol S ¡ 3.118 mol SO 2 - 10% = 2.806 mol SO 2<br />
-0.3118<br />
2.8062<br />
2.806 mol SO 2 ¡ 2.806 mol SO 3 - 10% = 2.525 mol SO 3<br />
-0.2806<br />
2.5254<br />
2.525 mol SO 3 ¡ 2.525 mol H 2 SO 4 - 10% = 2.273 mol H 2 SO 4<br />
-0.2525<br />
2.2725<br />
12.273 mol H 2 SO 4 2a 98.09 g b = 223.0 g H<br />
mol<br />
2 SO 4 formed<br />
13.118 mol S2a 1 mol H 2SO 4<br />
b = 3.118 mol H<br />
1 mol S<br />
2 SO 4 (theoretical yield)<br />
¢ 2.273 mol H 2SO 4<br />
3.118 mol H 2 SO 4<br />
≤11002 = 72.90% yield<br />
Alternate Solution:<br />
Calculation of yield. There are three chemical steps to the formation of H 2 SO 4 . Each step<br />
has a 10% loss of yield.<br />
Step 1:<br />
Step 2:<br />
Step 3:<br />
100% yield - 10% = 90.00% yield<br />
90.00% yield - 10% = 81.00% yield<br />
81.00% yield - 10% = 72.90% yield<br />
Now calculate the grams of product. One mole of sulfur will yield a maximum of 1 mol<br />
H 2 SO 4 . Therefore 3.118 mol S will give a maximum of 3.118 mol H 2 SO 4 .<br />
13.118 mol S2a 1 mol H 2SO 4<br />
1 mol S<br />
ba 98.09 g b10.72902 = 223.0 g H<br />
mol<br />
2 SO 4 yield<br />
53. According to the equations, the moles of CO 2 come from both reactions and the moles<br />
H 2 O come from only the first reaction.<br />
So the mol NaHCO 3 = 2 * mol H 2 O = 2 * 0.0357 mol = 0.0714 mol NaHCO 3<br />
10.0714 mol NaHCO 3 2a 84.01 g b = 6.00 g NaHCO<br />
mol<br />
3 in the sample<br />
110.00 g NaHCO 3 + Na 2 CO 3 2 - 6.00 g NaHCO 3 = 4.00 g Na 2 CO 3 in the sample<br />
- 115 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 116<br />
- <strong>Chapter</strong> 9 -<br />
a 6.00 g NaHCO 3<br />
b11002 = 60.0% NaHCO<br />
10.00 g<br />
3<br />
a 4.00 g Na 2CO 3<br />
b11002 = 40.0% Na<br />
10.00 g<br />
2 CO 3<br />
54. The balanced equation is 2 KClO 3 ¡ 2 KCl + 3 O 2<br />
12.82 g mixture - 9.45 g residue = 3.37 g O 2 lost by heating<br />
Because the O 2 lost came only from KClO 3 , we can use it to calculate the amount of<br />
KClO 3 in the mixture.<br />
The conversion is: g O 2 ¡ mol O 2 ¡ mol KClO 3 ¡ g KClO 3<br />
13.37 g O 2 2a 1 mol<br />
32.00 g ba2 mol KClO 3<br />
ba 122.6 g b = 8.61 g KClO<br />
3 mol O 2 mol<br />
3 in the mixture<br />
a 8.61 g KClO 3<br />
12.82 g sample b11002 = 67.2% KClO 3<br />
55. The balanced equation is<br />
Al(OH) 3 (s) + 3 HCl(aq) ¡ AlCl 3 (aq) + 3 H 2 O(l)<br />
The conversion is: L HCl ¡ g HCl ¡ mol HCl ¡ mol Al(OH) 3 : g Al(OH) 3<br />
a 2.5 L<br />
day<br />
g HCl<br />
ba3.0 ba 1 mol<br />
L<br />
36.46 g b ¢ 1 mol Al1OH2 3<br />
3 mol HCl<br />
≤ a 78.00 g b = 5.3 g Al1OH2<br />
mol<br />
3 >day<br />
Now calculate the number of 400. mg tablets that can be made from 5.3 g Al(OH) 3<br />
¢ 5.3 g Al(OH) 3 1000 mg<br />
≤ a ba 1 tablet<br />
day<br />
g 400. mg b = 13 tablets>day<br />
56.<br />
4 P + 5 O 2 ¡ P 4 O 10<br />
P 4 O 10 + 6 H 2 O ¡ 4 H 3 PO 4<br />
In the first reaction:<br />
120.0 g P2a 1 mol b = 0.646 mol P<br />
30.97 g<br />
130.0 g O 2 2a 1 mol<br />
32.00 g b = 0.938 mol O 2<br />
0.646 mol P 3.44 mol P<br />
This is a ratio of<br />
=<br />
0.938 mol O 2 5.00 mol O 2<br />
Therefore, P is the limiting reactant and the P 4 O 10 produced is:<br />
- 116 -
HEINS09-095-117v4.qxd 12/30/06 1:58 PM Page 117<br />
- <strong>Chapter</strong> 9 -<br />
10.646 mol P2¢ 1 mol P 4O 10<br />
4 mol P ≤ = 0.162 mol P 4O 10<br />
In the second reaction:<br />
115.0 g H 2 O2a 1 mol<br />
18.02 g b = 0.832 mol H 2O<br />
H 2 O 0.832 mol 5.14 mol<br />
and we have 0.162 mol P 4 O 10 . The ratio of is<br />
=<br />
P 4 O 10 0.162 mol 1.00 mol<br />
Therefore, H 2 O is the limiting reactant and the H 3 PO 4 produced is:<br />
10.832 mol H 2 O2¢ 4 mol H 3PO 4<br />
6 mol H 2 O ≤ a 97.99 g b = 54.4 g H<br />
mol<br />
3 PO 4<br />
- 117 -