Surface treatment of metals using an atmospheric pressure plasma ...
Surface treatment of metals using an atmospheric pressure plasma ...
Surface treatment of metals using an atmospheric pressure plasma ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
840 M.C. Kim et al. / <strong>Surface</strong> <strong>an</strong>d Coatings Technology 174 –175 (2003) 839–844<br />
from peak to peak <strong>an</strong>d 16–20 kHz, respectively. The<br />
length <strong>of</strong> <strong>plasma</strong> jet flame was approximately 30 mm<br />
<strong>an</strong>d the diameter was 10 mm, approximately. Mixed<br />
gases <strong>of</strong> N <strong>an</strong>d O with ultra-high purity (99.999%)<br />
2 2<br />
were used as the reactive gases with the ratios <strong>of</strong> N y 2<br />
O s4:1, (similar composition as air) <strong>an</strong>d N yO s1:4<br />
2 2 2<br />
under nearly <strong>atmospheric</strong> <strong>pressure</strong>. The gases were<br />
m<strong>an</strong>ually controlled by flow-controllers <strong>an</strong>d flowed const<strong>an</strong>tly<br />
into the jet-nozzle for the <strong>plasma</strong> ignition. The<br />
substrates were sequentially cle<strong>an</strong>ed in <strong>an</strong> acetone,<br />
methyl alcohol, <strong>an</strong>d de-ionized water by <strong>an</strong> ultrasonic<br />
cle<strong>an</strong>ing method. Al, SUS <strong>an</strong>d Cu were used for surface<br />
modification as the substrates. For more clear recognition<br />
by AFM <strong>an</strong>alysis, moreover, silicon (100) wafers<br />
that have more flat surfaces th<strong>an</strong> those <strong>of</strong> <strong>metals</strong> were<br />
also cle<strong>an</strong>ed by the same method <strong>an</strong>d treated in the<br />
<strong>plasma</strong> space under the same conditions. All <strong>treatment</strong>s<br />
were carried out at room temperature <strong>an</strong>d continuously<br />
performed under the conditions with the relative humidity<br />
<strong>of</strong> 64% for 8 days for the aging tests.<br />
In order to confirm the chemical <strong>an</strong>d physical reactions<br />
on the metal surfaces, X-ray photoelectron spectroscopy<br />
(XPS), field emission sc<strong>an</strong>ning electron<br />
microscopy (FE-SEM), <strong>an</strong>d atomic force microscopy<br />
(AFM) methods were used before <strong>an</strong>d after surface<br />
modification. For investigation <strong>of</strong> the <strong>plasma</strong> jet contained<br />
the chemical activated species in the <strong>plasma</strong><br />
space, optical emission spectroscopy (OES) was also<br />
used as optical diagnostic. The surface activities were,<br />
moreover, <strong>an</strong>alyzed by contact <strong>an</strong>gle <strong>an</strong>alyzer, which<br />
was also adopted in the <strong>an</strong>alysis <strong>of</strong> aging characteristics<br />
in sequence.<br />
3. Results <strong>an</strong>d discussion<br />
3.1. Contact <strong>an</strong>gle <strong>an</strong>alysis<br />
As the first step in our <strong>an</strong>alysis, we carried out the<br />
contact <strong>an</strong>gle measurements to obtain the optimum<br />
conditions, resulting in ch<strong>an</strong>ging to the high surface<br />
energy as the parameters <strong>of</strong> nozzle-surface gap <strong>an</strong>d<br />
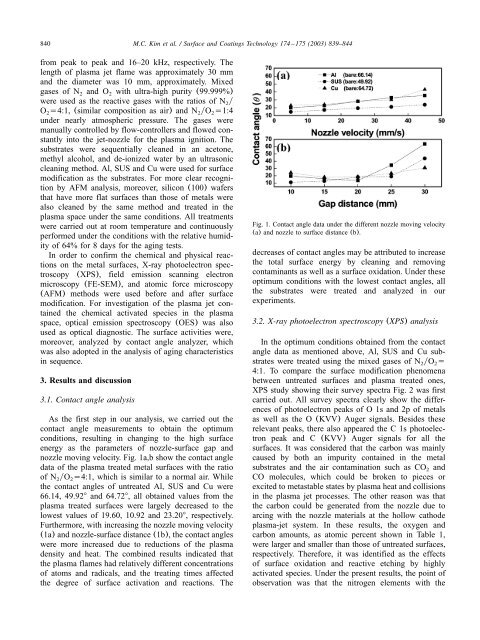
nozzle moving velocity. Fig. 1a,b show the contact <strong>an</strong>gle<br />
data <strong>of</strong> the <strong>plasma</strong> treated metal surfaces with the ratio<br />
<strong>of</strong> N2yO2s4:1, which is similar to a normal air. While<br />
the contact <strong>an</strong>gles <strong>of</strong> untreated Al, SUS <strong>an</strong>d Cu were<br />
66.14, 49.928 <strong>an</strong>d 64.728, all obtained values from the<br />
<strong>plasma</strong> treated surfaces were largely decreased to the<br />
lowest values <strong>of</strong> 19.60, 10.92 <strong>an</strong>d 23.208, respectively.<br />
Furthermore, with increasing the nozzle moving velocity<br />
(1a) <strong>an</strong>d nozzle-surface dist<strong>an</strong>ce (1b), the contact <strong>an</strong>gles<br />
were more increased due to reductions <strong>of</strong> the <strong>plasma</strong><br />
density <strong>an</strong>d heat. The combined results indicated that<br />
the <strong>plasma</strong> flames had relatively different concentrations<br />
<strong>of</strong> atoms <strong>an</strong>d radicals, <strong>an</strong>d the treating times affected<br />
the degree <strong>of</strong> surface activation <strong>an</strong>d reactions. The<br />
Fig. 1. Contact <strong>an</strong>gle data under the different nozzle moving velocity<br />
(a) <strong>an</strong>d nozzle to surface dist<strong>an</strong>ce (b).<br />
decreases <strong>of</strong> contact <strong>an</strong>gles may be attributed to increase<br />
the total surface energy by cle<strong>an</strong>ing <strong>an</strong>d removing<br />
contamin<strong>an</strong>ts as well as a surface oxidation. Under these<br />
optimum conditions with the lowest contact <strong>an</strong>gles, all<br />
the substrates were treated <strong>an</strong>d <strong>an</strong>alyzed in our<br />
experiments.<br />
3.2. X-ray photoelectron spectroscopy (XPS) <strong>an</strong>alysis<br />
In the optimum conditions obtained from the contact<br />
<strong>an</strong>gle data as mentioned above, Al, SUS <strong>an</strong>d Cu substrates<br />
were treated <strong>using</strong> the mixed gases <strong>of</strong> N2yO2s<br />
4:1. To compare the surface modification phenomena<br />
between untreated surfaces <strong>an</strong>d <strong>plasma</strong> treated ones,<br />
XPS study showing their survey spectra Fig. 2 was first<br />
carried out. All survey spectra clearly show the differences<br />
<strong>of</strong> photoelectron peaks <strong>of</strong> O 1s <strong>an</strong>d 2p <strong>of</strong> <strong>metals</strong><br />
as well as the O (KVV) Auger signals. Besides these<br />
relev<strong>an</strong>t peaks, there also appeared the C 1s photoelectron<br />
peak <strong>an</strong>d C (KVV) Auger signals for all the<br />
surfaces. It was considered that the carbon was mainly<br />
caused by both <strong>an</strong> impurity contained in the metal<br />
substrates <strong>an</strong>d the air contamination such as CO2<br />
<strong>an</strong>d<br />
CO molecules, which could be broken to pieces or<br />
excited to metastable states by <strong>plasma</strong> heat <strong>an</strong>d collisions<br />
in the <strong>plasma</strong> jet processes. The other reason was that<br />
the carbon could be generated from the nozzle due to<br />
arcing with the nozzle materials at the hollow cathode<br />
<strong>plasma</strong>-jet system. In these results, the oxygen <strong>an</strong>d<br />
carbon amounts, as atomic percent shown in Table 1,<br />
were larger <strong>an</strong>d smaller th<strong>an</strong> those <strong>of</strong> untreated surfaces,<br />
respectively. Therefore, it was identified as the effects<br />
<strong>of</strong> surface oxidation <strong>an</strong>d reactive etching by highly<br />
activated species. Under the present results, the point <strong>of</strong><br />
observation was that the nitrogen elements with the