ayout 1 - EMBL Grenoble
ayout 1 - EMBL Grenoble
ayout 1 - EMBL Grenoble
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
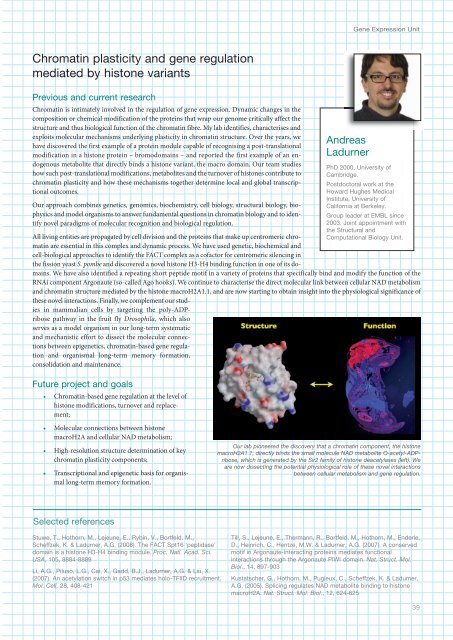
Gene Expression UnitChromatin plasticity and gene regulationmediated by histone variantsPrevious and current researchChromatin is intimately involved in the regulation of gene expression. Dynamic changes in thecomposition or chemical modification of the proteins that wrap our genome critically affect thestructure and thus biological function of the chromatin fibre. My lab identifies, characterises andexploits molecular mechanisms underlying plasticity in chromatin structure. Over the years, wehave discovered the first example of a protein module capable of recognising a post-translationalmodification in a histone protein – bromodomains – and reported the first example of an endogenousmetabolite that directly binds a histone variant, the macro domain. Our team studieshow such post-translational modifications, metabolites and the turnover of histones contribute tochromatin plasticity and how these mechanisms together determine local and global transcriptionaloutcomes.Our approach combines genetics, genomics, biochemistry, cell biology, structural biology, biophysicsand model organisms to answer fundamental questions in chromatin biology and to identifynovel paradigms of molecular recognition and biological regulation.AndreasLadurnerPhD 2000, University ofCambridge.Postdoctoral work at theHoward Hughes MedicalInstitute, University ofCalifornia at Berkeley.Group leader at <strong>EMBL</strong> since2003. Joint appointment withthe Structural andComputational Biology Unit.All living entities are propagated by cell division and the proteins that make up centromeric chromatinare essential in this complex and dynamic process. We have used genetic, biochemical andcell-biological approaches to identify the FACT complex as a cofactor for centromeric silencing inthe fission yeast S. pombe and discovered a novel histone H3-H4 binding function in one of its domains.We have also identified a repeating short peptide motif in a variety of proteins that specifically bind and modify the function of theRNAi component Argonaute (so-called Ago hooks). We continue to characterise the direct molecular link between cellular NAD metabolismand chromatin structure mediated by the histone macroH2A1.1, and are now starting to obtain insight into the physiological significance ofthese novel interactions. Finally, we complement our studiesin mammalian cells by targeting the poly-ADPribosepathway in the fruit fly Drosophila, which alsoserves as a model organism in our long-term systematicand mechanistic effort to dissect the molecular connectionsbetween epigenetics, chromatin-based gene regulationand organismal long-term memory formation,consolidation and maintenance.Future project and goals• Chromatin-based gene regulation at the level ofhistone modifications, turnover and replacement;• Molecular connections between histonemacroH2A and cellular NAD metabolism;• High-resolution structure determination of keychromatin plasticity components;• Transcriptional and epigenetic basis for organismallong-term memory formation.Our lab pioneered the discovery that a chromatin component, the histonemacroH2A1.1, directly binds the small molecule NAD metabolite O-acetyl-ADPribose,which is generated by the Sir2 family of histone deacetylases (left). Weare now dissecting the potential physiological role of these novel interactionsbetween cellular metabolism and gene regulation.Selected referencesStuwe, T., Hothorn, M., Lejeune, E., Rybin, V., Bortfeld, M.,Scheffzek, K. & Ladurner, A.G. (2008). The FACT Spt16 ‘peptidase’domain is a histone H3-H binding module. Proc. Natl. Acad. Sci.USA, 105, 888-8889Li, A.G., Piluso, L.G., Cai, X., Gadd, B.J., Ladurner, A.G. & Liu, X.(2007). An acetylation switch in p53 mediates holo-TFIID recruitment.Mol. Cell, 28, 08-21Till, S., Lejeune, E., Thermann, R., Bortfeld, M., Hothorn, M., Enderle,D., Heinrich, C., Hentze, M.W. & Ladurner, A.G. (2007). A conservedmotif in Argonaute-interacting proteins mediates functionalinteractions through the Argonaute PIWI domain. Nat. Struct. Mol.Biol., 1, 897-903Kustatscher, G., Hothorn, M., Pugieux, C., Scheffzek, K. & Ladurner,A.G. (2005). Splicing regulates NAD metabolite binding to histonemacroH2A. Nat. Struct. Mol. Biol., 12, 62-62539