Cortical reorganization in motor cortex after graft of both hands
Cortical reorganization in motor cortex after graft of both hands
Cortical reorganization in motor cortex after graft of both hands
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
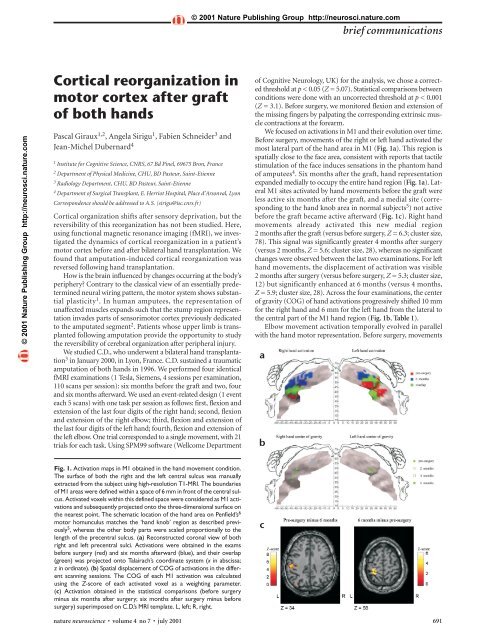
© 2001 Nature Publish<strong>in</strong>g Group http://neurosci.nature.combrief communications© 2001 Nature Publish<strong>in</strong>g Group http://neurosci.nature.com<strong>Cortical</strong> <strong>reorganization</strong> <strong>in</strong><strong>motor</strong> <strong>cortex</strong> <strong>after</strong> <strong>graft</strong><strong>of</strong> <strong>both</strong> <strong>hands</strong>Pascal Giraux 1,2 , Angela Sirigu 1 , Fabien Schneider 3 andJean-Michel Dubernard 41 Institute for Cognitive Science, CNRS, 67 Bd P<strong>in</strong>el, 69675 Bron, France2 Department <strong>of</strong> Physical Medic<strong>in</strong>e, CHU, BD Pasteur, Sa<strong>in</strong>t-Etienne3 Radiology Department, CHU, BD Pasteur, Sa<strong>in</strong>t-Etienne4 Department <strong>of</strong> Surgical Transplant, E. Herriot Hospital, Place d’Arsonval, LyonCorrespondence should be addressed to A.S. (sirigu@isc.cnrs.fr)<strong>Cortical</strong> organization shifts <strong>after</strong> sensory deprivation, but thereversibility <strong>of</strong> this <strong>reorganization</strong> has not been studied. Here,us<strong>in</strong>g functional magnetic resonance imag<strong>in</strong>g (fMRI), we <strong>in</strong>vestigatedthe dynamics <strong>of</strong> cortical <strong>reorganization</strong> <strong>in</strong> a patient’s<strong>motor</strong> <strong>cortex</strong> before and <strong>after</strong> bilateral hand transplantation. Wefound that amputation-<strong>in</strong>duced cortical <strong>reorganization</strong> wasreversed follow<strong>in</strong>g hand transplantation.How is the bra<strong>in</strong> <strong>in</strong>fluenced by changes occurr<strong>in</strong>g at the body’speriphery? Contrary to the classical view <strong>of</strong> an essentially predeterm<strong>in</strong>edneural wir<strong>in</strong>g pattern, the <strong>motor</strong> system shows substantialplasticity 1 . In human amputees, the representation <strong>of</strong>unaffected muscles expands such that the stump region representation<strong>in</strong>vades parts <strong>of</strong> sensori<strong>motor</strong> <strong>cortex</strong> previously dedicatedto the amputated segment 2 . Patients whose upper limb is transplantedfollow<strong>in</strong>g amputation provide the opportunity to studythe reversibility <strong>of</strong> cerebral organization <strong>after</strong> peripheral <strong>in</strong>jury.We studied C.D., who underwent a bilateral hand transplantation3 <strong>in</strong> January 2000, <strong>in</strong> Lyon, France. C.D. susta<strong>in</strong>ed a traumaticamputation <strong>of</strong> <strong>both</strong> <strong>hands</strong> <strong>in</strong> 1996. We performed four identicalfMRI exam<strong>in</strong>ations (1 Tesla, Siemens, 4 sessions per exam<strong>in</strong>ation,110 scans per session): six months before the <strong>graft</strong> and two, fourand six months <strong>after</strong>ward. We used an event-related design (1 eventeach 5 scans) with one task per session as follows: first, flexion andextension <strong>of</strong> the last four digits <strong>of</strong> the right hand; second, flexionand extension <strong>of</strong> the right elbow; third, flexion and extension <strong>of</strong>the last four digits <strong>of</strong> the left hand; fourth, flexion and extension <strong>of</strong>the left elbow. One trial corresponded to a s<strong>in</strong>gle movement, with 21trials for each task. Us<strong>in</strong>g SPM99 s<strong>of</strong>tware (Wellcome Department<strong>of</strong> Cognitive Neurology, UK) for the analysis, we chose a correctedthreshold at p < 0.05 (Z = 5.07). Statistical comparisons betweenconditions were done with an uncorrected threshold at p < 0.001(Z = 3.1). Before surgery, we monitored flexion and extension <strong>of</strong>the miss<strong>in</strong>g f<strong>in</strong>gers by palpat<strong>in</strong>g the correspond<strong>in</strong>g extr<strong>in</strong>sic musclecontractions at the forearm.We focused on activations <strong>in</strong> M1 and their evolution over time.Before surgery, movements <strong>of</strong> the right or left hand activated themost lateral part <strong>of</strong> the hand area <strong>in</strong> M1 (Fig. 1a). This region isspatially close to the face area, consistent with reports that tactilestimulation <strong>of</strong> the face <strong>in</strong>duces sensations <strong>in</strong> the phantom hand<strong>of</strong> amputees 4 . Six months <strong>after</strong> the <strong>graft</strong>, hand representationexpanded medially to occupy the entire hand region (Fig. 1a). LateralM1 sites activated by hand movements before the <strong>graft</strong> wereless active six months <strong>after</strong> the <strong>graft</strong>, and a medial site (correspond<strong>in</strong>gto the hand knob area <strong>in</strong> normal subjects 5 ) not activebefore the <strong>graft</strong> became active <strong>after</strong>ward (Fig. 1c). Right handmovements already activated this new medial region2 months <strong>after</strong> the <strong>graft</strong> (versus before surgery, Z = 6.3; cluster size,78). This signal was significantly greater 4 months <strong>after</strong> surgery(versus 2 months, Z = 5.6; cluster size, 28), whereas no significantchanges were observed between the last two exam<strong>in</strong>ations. For lefthand movements, the displacement <strong>of</strong> activation was visible2 months <strong>after</strong> surgery (versus before surgery, Z = 5.3; cluster size,12) but significantly enhanced at 6 months (versus 4 months,Z = 5.9; cluster size, 28). Across the four exam<strong>in</strong>ations, the center<strong>of</strong> gravity (COG) <strong>of</strong> hand activations progressively shifted 10 mmfor the right hand and 6 mm for the left hand from the lateral tothe central part <strong>of</strong> the M1 hand region (Fig. 1b, Table 1).Elbow movement activation temporally evolved <strong>in</strong> parallelwith the hand <strong>motor</strong> representation. Before surgery, movementsabFig. 1. Activation maps <strong>in</strong> M1 obta<strong>in</strong>ed <strong>in</strong> the hand movement condition.The surface <strong>of</strong> <strong>both</strong> the right and the left central sulcus was manuallyextracted from the subject us<strong>in</strong>g high-resolution T1-MRI. The boundaries<strong>of</strong> M1 areas were def<strong>in</strong>ed with<strong>in</strong> a space <strong>of</strong> 6 mm <strong>in</strong> front <strong>of</strong> the central sulcus.Activated voxels with<strong>in</strong> this def<strong>in</strong>ed space were considered as M1 activationsand subsequently projected onto the three-dimensional surface onthe nearest po<strong>in</strong>t. The schematic location <strong>of</strong> the hand area on Penfield’s 6<strong>motor</strong> homunculus matches the ‘hand knob’ region as described previously5 , whereas the other body parts were scaled proportionally to thelength <strong>of</strong> the precentral sulcus. (a) Reconstructed coronal view <strong>of</strong> <strong>both</strong>right and left precentral sulci. Activations were obta<strong>in</strong>ed <strong>in</strong> the examsbefore surgery (red) and six months <strong>after</strong>ward (blue), and their overlap(green) was projected onto Talairach’s coord<strong>in</strong>ate system (x <strong>in</strong> abscissa;z <strong>in</strong> ord<strong>in</strong>ate). (b) Spatial displacement <strong>of</strong> COG <strong>of</strong> activations <strong>in</strong> the differentscann<strong>in</strong>g sessions. The COG <strong>of</strong> each M1 activation was calculatedus<strong>in</strong>g the Z-score <strong>of</strong> each activated voxel as a weight<strong>in</strong>g parameter.(c) Activation obta<strong>in</strong>ed <strong>in</strong> the statistical comparisons (before surgerym<strong>in</strong>us six months <strong>after</strong> surgery; six months <strong>after</strong> surgery m<strong>in</strong>us beforesurgery) superimposed on C.D.’s MRI template. L, left; R, right.cnature neuroscience • volume 4 no 7 • july 2001 691
ief communications© 2001 Nature Publish<strong>in</strong>g Group http://neurosci.nature.com© 2001 Nature Publish<strong>in</strong>g Group http://neurosci.nature.comTable 1. Time course <strong>of</strong> the center <strong>of</strong> gravity <strong>of</strong> M1 activations for the hand and elbow, for the different exams.Movement Before surgery 2 months 4 months 6 months Change <strong>in</strong> distance (before surgeryx y z x y z x y z x y z versus 6 months <strong>after</strong>ward)Right hand –42 –22 40 –40 –25 46 –39 –25 45 –39 –27 48 10 mmLeft hand 36 –18 46 33 –23 49 38 –21 48 33 –22 49 6 mmRight elbow –34 –29 49 –26 –33 54 –30 –31 52 –28 –32 54 8 mmLeft elbow 31 –23 47 29 –28 51 30 –26 52 30 –26 54 7 mmValues are <strong>in</strong> Talairach coord<strong>in</strong>ates.<strong>of</strong> either elbow activated a contralateral central region <strong>of</strong> M1 thatcorresponds to the hand <strong>motor</strong> map (Fig. 2a). Six months <strong>after</strong>ward,elbow activations migrated toward the upper part <strong>of</strong> thelimb representation, classically def<strong>in</strong>ed as the arm region 6 . DifferentM1 cortical maps were observed before and six months<strong>after</strong> surgery: before the <strong>graft</strong>, a central region, and <strong>after</strong>ward, anew superior medial area (Fig. 2c). As for hand movements, thenew superior locus <strong>of</strong> activation was apparent at two months (versusbefore surgery; Z > 10; cluster size, 236) for right elbow movements.The signal strength had <strong>in</strong>creased significantly at 4 months(versus 2 months; Z = 5.2; cluster size, 26) but did not <strong>in</strong>creasefurther at 6 months. For left elbow movements, the most significantsignal changes occurred between 4 and 6 months (Z = 6.9;cluster size, 67). The COGs along the four exam<strong>in</strong>ations underwenta progressive shift from the central to the superior part <strong>of</strong>M1 (right elbow, 8 mm; left elbow, 7 mm; Fig. 2b; Table 1).The changes observed with<strong>in</strong> the <strong>motor</strong> <strong>cortex</strong> for hand andelbow representations seemed to be strongly correlated <strong>in</strong> <strong>both</strong>abtime and space. Across the different exam<strong>in</strong>ations, the distancebetween COGs rema<strong>in</strong>ed fairly constant for the two types <strong>of</strong>movements (Table 1). Hand and elbow activations showed a highdegree <strong>of</strong> overlap, the extent <strong>of</strong> which <strong>in</strong>creased from beforesurgery through the exam<strong>in</strong>ations <strong>after</strong> surgery.Parallel changes were also recorded <strong>in</strong> somatosensory <strong>cortex</strong>.Hand and elbow movements activated S1 <strong>in</strong> all four exam<strong>in</strong>ationsand their pattern was similar to that <strong>of</strong> the <strong>motor</strong> <strong>cortex</strong> activations.These results show that <strong>graft</strong>ed <strong>hands</strong> come to be recognizedand activated normally by sensori<strong>motor</strong> <strong>cortex</strong>. The displacement<strong>of</strong> the cortical activity from lateral to medial along the precentralgyrus is remarkably similar for hand and elbowmovements. These cortical maps covered <strong>in</strong> the same amount <strong>of</strong>time a similar distance, as revealed by the trajectory <strong>of</strong> the activationCOGs. This suggests that new peripheral <strong>in</strong>puts alloweda global remodel<strong>in</strong>g <strong>of</strong> the limb cortical map and reversed thefunctional <strong>reorganization</strong> <strong>in</strong>duced by the amputation. The spatialtrajectory <strong>of</strong> these activations <strong>in</strong> time further <strong>in</strong>dicates that thecortical rearrangement occurs <strong>in</strong> an orderly manner; the handand arm representations tend to return to their orig<strong>in</strong>al corticallocus. Hence, bra<strong>in</strong> plasticity is accomplished with reference toa previous pre-amputation somatotopic body representation.What are the mechanisms underly<strong>in</strong>g this cortical reversibility?In monkeys with amputated segments, severed efferentmotoneurons preserve their functional efficacy by target<strong>in</strong>g newmuscles 7 . As efferent and afferent central pathway neurons survive<strong>after</strong> they are cut, the sensori<strong>motor</strong> circuit may be functionallyready <strong>after</strong> the <strong>graft</strong>; this could expla<strong>in</strong> why activity shifts areobserved as early as two months <strong>after</strong> surgery. The <strong>in</strong>tr<strong>in</strong>sicchanges with<strong>in</strong> M1 may result from a shift <strong>in</strong> the strengths <strong>of</strong> activationsamong exist<strong>in</strong>g connections. If we assume that hand andelbow had preexist<strong>in</strong>g connections, the elbow activation <strong>in</strong> thephase before surgery may emerge as a change <strong>in</strong> the weight <strong>of</strong>these connections; that is, the elbow connection may be enhancedat the expense <strong>of</strong> the deprived hand region. The hand transplantmay have restored the efficacy <strong>of</strong> the orig<strong>in</strong>al connections at theexpense, this time, <strong>of</strong> the elbow representation, thus allow<strong>in</strong>g typicalfeatures <strong>of</strong> cortical organization to reappear <strong>in</strong> the <strong>motor</strong> map.cACKNOWLEDGEMENTSThis work was supported by a CNRS grant to A.S.RECEIVED 5 FEBRUARY; ACCEPTED 30 APRIL 2001Fig. 2. Activation maps <strong>in</strong> M1 obta<strong>in</strong>ed <strong>in</strong> the elbow movement condition.(a–c) Same as <strong>in</strong> Fig.1.1. Kaas, J. H. <strong>in</strong> The Organization <strong>of</strong> the Cerebral Cortex (eds. Schmitt, F. O.,Worden, F. G., Adelman, G. & Dennis, S. G.) 223–236 (MIT Press,Cambridge, Massachusetts, 1981).2. Roricht S., Meyer, B. U., Niehaus, L. & Brandt, S. A. Neurology 53, 106–111(1999).3. Dubernard J. M. et al. Lancet 353, 1315–1320 (1999).4. Ramachandran, V. S., Rogers-Ramachandran, D. & Stewart, M. Science 258,1159–1160 (1992).5. Yousry, T. A. et al. Bra<strong>in</strong> 120, 141–157 (1997).6. Penfield W. The Cerebral Cortex <strong>of</strong> the Man (MacMillan, New York, 1950).7. Wu, C. W. & Kaas, J. H. Neuron 28, 967–978 (2000).692 nature neuroscience • volume 4 no 7 • july 2001