STUDY GUIDE
STUDY GUIDE
STUDY GUIDE
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
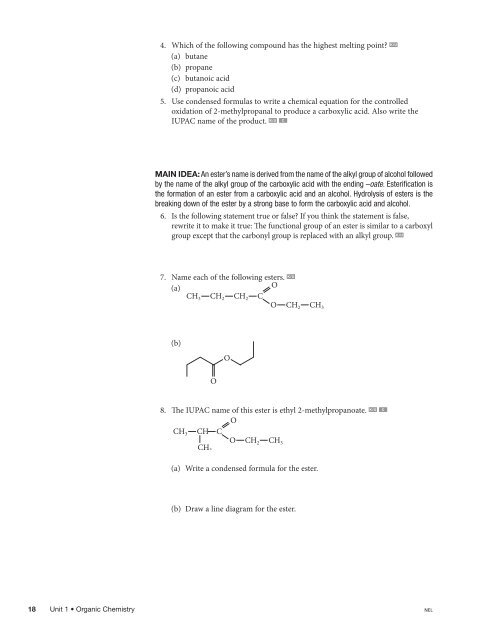
4. Which of the following compound has the highest melting point? K/U(a) butane(b) propane(c) butanoic acid(d) propanoic acid5. Use condensed formulas to write a chemical equation for the controlledoxidation of 2-methylpropanal to produce a carboxylic acid. Also write theIUPAC name of the product. K/U CMAIN IDEA: An ester’s name is derived from the name of the alkyl group of alcohol followedby the name of the alkyl group of the carboxylic acid with the ending –oate. Esterification isthe formation of an ester from a carboxylic acid and an alcohol. Hydrolysis of esters is thebreaking down of the ester by a strong base to form the carboxylic acid and alcohol.6. Is the following statement true or false? If you think the statement is false,rewrite it to make it true: The functional group of an ester is similar to a carboxylgroup except that the carbonyl group is replaced with an alkyl group. K/U7. Name each of the following esters. K/U(a)OCH 3 CH 2 CH 2 CO CH 2 CH 3(b)OO8. The IUPAC name of this ester is ethyl 2-methylpropanoate. K/U COCH 3 CH CCH 3O CH 2 CH 3C01-F35-OC12USG.ai(a) Write a condensed formula for the ester.(b) Draw a line diagram for the ester.C01-F36-OC12USG.aiC01-F37-OC12USG.ai18Biology 12 Study Guide0176520848Unit 1 • Organic Chemistry NELFigure Number C01-F35-OC12USG.aiCompanyMPS