Chapter 1 Gas Power Cycle
Chapter 1 Gas Power Cycle
Chapter 1 Gas Power Cycle
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
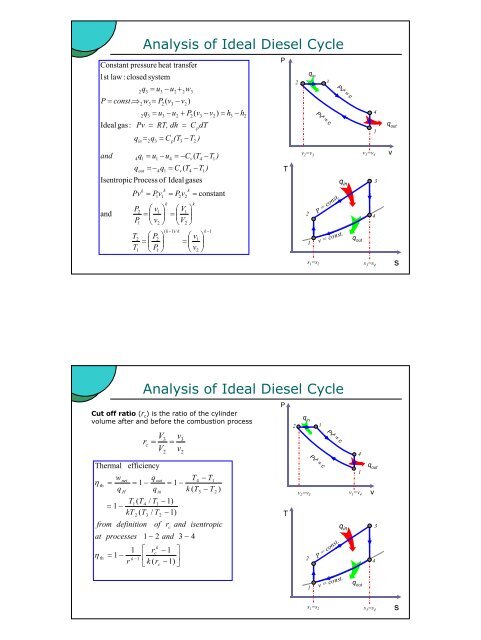
1st law : closed system<br />
Ideal gas:<br />
and<br />
Analysis of Ideal Diesel <strong>Cycle</strong><br />
Constant pressure heat transfer<br />
2<br />
P = const.<br />
⇒ w = P ( v − v )<br />
and<br />
2<br />
2<br />
4 1<br />
3<br />
3<br />
3<br />
2<br />
= − q = C ( T −T<br />
)<br />
Isentropic Process of Ideal gases<br />
3<br />
1<br />
4<br />
3<br />
2<br />
3<br />
1<br />
k<br />
1v1<br />
4<br />
3<br />
Pv = RT, dh = C dT<br />
q = q = C (T −T<br />
)<br />
in<br />
2<br />
2<br />
q = u − u = −C<br />
( T −T<br />
)<br />
q<br />
q = u − u + w<br />
out<br />
k<br />
Pv = P<br />
k<br />
p<br />
3<br />
v<br />
= P<br />
2<br />
2<br />
4<br />
2<br />
k<br />
2v2<br />
P ⎛ 2 v ⎞ ⎛ 1 V ⎞ 1<br />
= ⎜<br />
⎟ = ⎜<br />
⎟<br />
P1<br />
⎝ v2<br />
⎠ ⎝V2<br />
⎠<br />
( k −1)<br />
/ k<br />
T ⎛ ⎞ ⎛ ⎞<br />
2 P2<br />
v1<br />
= ⎜<br />
⎟ = ⎜<br />
⎟<br />
T1<br />
⎝ P1<br />
⎠ ⎝ v2<br />
⎠<br />
3<br />
q = u − u + P ( v − v ) = h − h<br />
v<br />
2<br />
3<br />
4<br />
1<br />
= constant<br />
k<br />
p<br />
k −1<br />
2<br />
1<br />
3<br />
2<br />
P<br />
T<br />
2<br />
q in<br />
v 2 =v 3<br />
2<br />
1<br />
s 1 =s 2<br />
3<br />
Pv k = c<br />
P = const.<br />
Pv k = c<br />
q in<br />
v = const.<br />
Analysis of Ideal Diesel <strong>Cycle</strong><br />
Cut off ratio (r c ) is the ratio of the cylinder<br />
volume after and before the combustion process<br />
Thermal<br />
w<br />
η th =<br />
q<br />
from<br />
at<br />
net<br />
H<br />
r c<br />
efficiency<br />
q<br />
= 1 −<br />
q<br />
V3<br />
v3<br />
= =<br />
V v<br />
T1<br />
( T4<br />
/ T1<br />
− 1)<br />
= 1 −<br />
kT ( T / T − 1)<br />
2<br />
definition<br />
processes<br />
3<br />
out<br />
in<br />
of<br />
2<br />
2<br />
1 − 2 and 3 − 4<br />
2<br />
T4<br />
− T1<br />
= 1 −<br />
k ( T − T )<br />
r<br />
c<br />
and<br />
k<br />
1 ⎡ r ⎤<br />
c − 1<br />
η th = 1 − k −1<br />
⎢ ⎥<br />
r ⎣ k ( rc<br />
− 1)<br />
⎦<br />
3<br />
2<br />
isentropic<br />
P<br />
T<br />
2<br />
q in<br />
v 2 =v 3<br />
2<br />
3<br />
Pv k = c<br />
1<br />
s 1 =s 2<br />
Pv k = c<br />
P = const.<br />
q in<br />
v = const.<br />
q out<br />
4<br />
1<br />
v 1 =v 4<br />
q out<br />
4<br />
1<br />
v 1 =v 4<br />
4<br />
3<br />
s3 =s 4<br />
q out<br />
v<br />
4<br />
3<br />
s3 =s 4<br />
q out<br />
v<br />
s<br />
s