Chapter 1 Gas Power Cycle
Chapter 1 Gas Power Cycle
Chapter 1 Gas Power Cycle
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
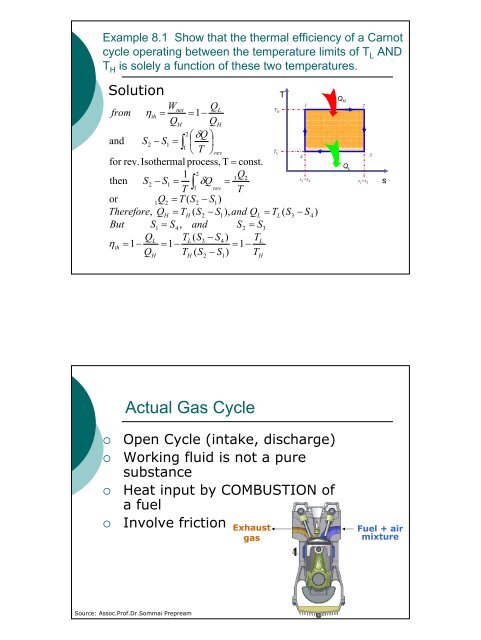
Example 8.1 Show that the thermal efficiency of a Carnot<br />
cycle operating between the temperature limits of T L AND<br />
T H is solely a function of these two temperatures.<br />
Solution<br />
from<br />
Wnet<br />
QL<br />
ηth<br />
= = 1−<br />
QH<br />
QH<br />
TH 1<br />
2⎛<br />
δQ<br />
⎞<br />
and S2<br />
− S1<br />
= ∫ ⎜ ⎟<br />
1 ⎝ T ⎠rev<br />
for rev. Isothermal process, T = const.<br />
TL 4<br />
1 2<br />
1Q2<br />
then S2<br />
− S1<br />
= Q<br />
T ∫ δ =<br />
1 rev T<br />
or<br />
1Q2<br />
= T ( S2<br />
− S1)<br />
Therefore,<br />
QH<br />
= TH<br />
( S2<br />
− S1),<br />
and QL<br />
= TL<br />
( S3<br />
− S4<br />
)<br />
But S1<br />
= S4,<br />
and S2<br />
= S3<br />
QL<br />
TL<br />
( S3<br />
− S4<br />
) TL<br />
ηth<br />
= 1−<br />
= 1−<br />
= 1−<br />
Q T ( S − S ) T<br />
H<br />
H<br />
Actual <strong>Gas</strong> <strong>Cycle</strong><br />
2<br />
1<br />
H<br />
T<br />
Q H<br />
Q L<br />
s 1 =s 4 s2 =s 3<br />
� Open <strong>Cycle</strong> (intake, discharge)<br />
� Working fluid is not a pure<br />
substance<br />
� Heat input by COMBUSTION of<br />
a fuel<br />
� Involve friction<br />
Source: Assoc.Prof.Dr.Sommai Prepream<br />
Exhaust<br />
gas<br />
2<br />
3<br />
s<br />
Fuel + air<br />
mixture