Chapter 1 Gas Power Cycle
Chapter 1 Gas Power Cycle
Chapter 1 Gas Power Cycle
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Example 8.2 An ideal Otto cycle has a compression ratio of 8. At the<br />
begining of the compression process, the air is at 100 kPa and 17 o C,<br />
and 800 kJ/kg of heat is transfered to air during the heat addition<br />
proceed. Accounting for the variation of specific heats of air with<br />
temperature, determine, (a) the maximum temperature and pressure<br />
which occur during the cycle, (b) the net work out put, (c) the thermal<br />
efficiency, and (d) the mean effective pressure of the cycle<br />
Given:<br />
rv = 8.0<br />
P1 = 100 kPa and<br />
T1 =17oC qH = 800 kJ/kg<br />
variation of specific<br />
heats<br />
Determine:<br />
a) T max<br />
b) w net<br />
c) η th<br />
d) MEP<br />
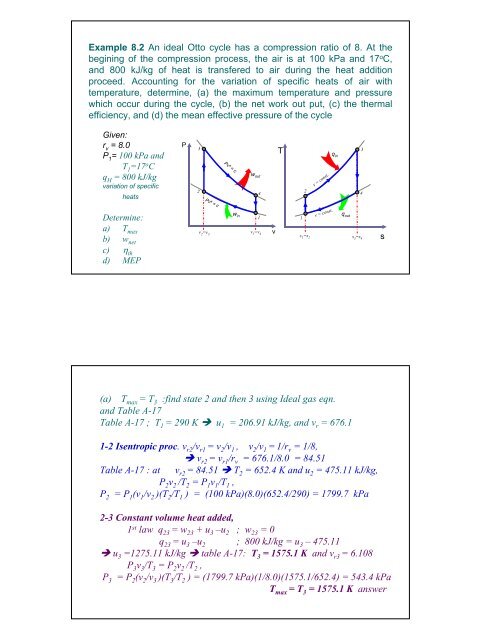
P<br />
3<br />
2<br />
v 2 =v 3<br />
Pv k = c<br />
Pv k = c<br />
4<br />
1<br />
v 1 =v 4<br />
v<br />
T<br />
1<br />
2<br />
s 1 =s 2<br />
v = const.<br />
v = const.<br />
(a) T max = T 3 :find state 2 and then 3 using Ideal gas eqn.<br />
and Table A-17<br />
Table A-17 ; T 1 = 290 K � u 1 = 206.91 kJ/kg, and v r = 676.1<br />
w in<br />
1-2 Isentropic proc. v r2/v r1 = v 2/v 1 , v 2/v 1 =1/r v = 1/8,<br />
� v r2 = v r1/r v = 676.1/8.0 = 84.51<br />
Table A-17 : at v r2 = 84.51 � T 2 = 652.4 K and u 2 = 475.11 kJ/kg,<br />
P 2v 2 /T 2 = P 1v 1/T 1 ,<br />
P 2 = P 1(v 1/v 2 )(T 2/T 1 ) = (100 kPa)(8.0)(652.4/290) = 1799.7 kPa<br />
2-3 Constant volume heat added,<br />
1 st law q 23 = w 23 + u 3 –u 2 ; w 23 = 0<br />
q 23 = u 3 –u 2 ; 800 kJ/kg = u 3 – 475.11<br />
� u 3 =1275.11 kJ/kg � table A-17: T 3 = 1575.1 K and v r3 = 6.108<br />
P 3v 3/T 3 = P 2v 2 /T 2 ,<br />
P 3 = P 2(v 2/v 3 )(T 3/T 2 ) = (1799.7 kPa)(1/8.0)(1575.1/652.4) = 543.4 kPa<br />
T max =T 3 = 1575.1 K answer<br />
w out<br />
q in<br />
q out<br />
3<br />
4<br />
s3 =s 4<br />
s