Statistical Physics
Statistical Physics
Statistical Physics
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
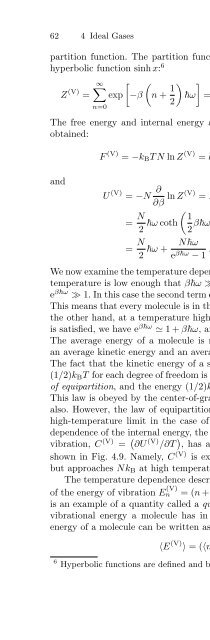
62 4 Ideal Gasespartition function. The partition function can be expressed in terms of thehyperbolic function sinh x: 6∞∑[ (Z (V) = exp −β n + 1 ) ]ω = e−βω/22 1 − e −βω = 12sinh(βω/2) . (4.46)n=0The free energy and internal energy associated with vibration can then beobtained:[ ( )] 1F (V) = −k B TN ln Z (V) = k B TN ln 2sinh2 βω (4.47)andU (V) = −N ∂∂β ln Z(V) = N ∂ [ ( )] 1∂β ln 2sinh2 βω= N ( ) 12 ω coth 2 βω= N Nωω +2 e βω − 1 . (4.48)We now examine the temperature dependence of the internal energy. When thetemperature is low enough that βω ≫ 1, or ω ≫ k B T , is satisfied, we havee βω ≫ 1. In this case the second term can be neglected, and U (V) =(N/2)ω.This means that every molecule is in the ground state at low temperature. Onthe other hand, at a temperature high enough that βω ≪ 1, or ω ≪ k B T ,is satisfied, we have e βω ≃ 1+βω, andU (V) ≃ (N/2)ω + Nk B T ≃ Nk B T .The average energy of a molecule is now k B T ; namely, every molecule hasan average kinetic energy and an average potential energy of (1/2)k B T each.The fact that the kinetic energy of a system at high temperature is equal to(1/2)k B T for each degree of freedom is known empirically and is called the lawof equipartition, and the energy (1/2)k B T is often called the thermal energy.This law is obeyed by the center-of-gravity part of the internal energy (4.41)also. However, the law of equipartition is obeyed only approximately in thehigh-temperature limit in the case of vibration. Owing to the temperaturedependence of the internal energy, the contribution to the heat capacity fromvibration, C (V) = ( ∂U (V) /∂T ) , has a temperature dependence of the formshown in Fig. 4.9. Namely, C (V) is exponentially small at low temperature,but approaches Nk B at high temperature.The temperature dependence described above arises from the discretenessof the energy of vibration E n(V) =(n +1/2)ω. Theintegern in this equationis an example of a quantity called a quantum number. It indicates how muchvibrational energy a molecule has in units of ω. The average vibrationalenergy of a molecule can be written as〈E (V) 〉 =(〈n〉 +1/2)ω, (4.49)6 Hyperbolic functions are defined and briefly described in Appendix E.









![Práctica [PDF] - Universidad de Carabobo, FACYT - computacion](https://img.yumpu.com/48491415/1/190x245/practica-pdf-universidad-de-carabobo-facyt-computacion.jpg?quality=85)