Statistical Physics
Statistical Physics
Statistical Physics
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
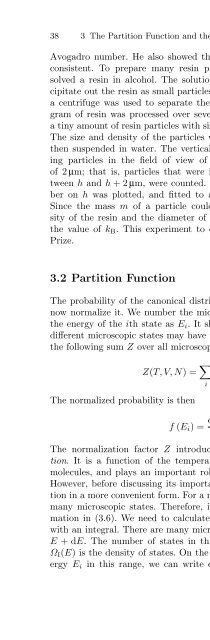
38 3 The Partition Function and the Free EnergyAvogadro number. He also showed that the values of these quantities wereconsistent. To prepare many resin particles of the same size, Perrin dissolveda resin in alcohol. The solution was then mixed with water to precipitateout the resin as small particles. Since the particles had various sizes,a centrifuge was used to separate the particles according to size. One kilogramof resin was processed over several months, and Perrin obtained onlya tiny amount of resin particles with sizes that were sufficiently well matched.The size and density of the particles were measured, and the particles werethen suspended in water. The vertical distribution was measured by countingparticles in the field of view of a microscope with a depth of focusof 2 µm; that is, particles that were in focus, which floated at heights betweenh and h +2µm, were counted. The dependence of the resulting numberon h was plotted, and fitted to a dependence on h of the form (3.5).Since the mass m of a particle could be determined from the mass densityof the resin and the diameter of the particles, this fitting process gavethe value of k B . This experiment to determine k B brought Perrin a NobelPrize.3.2 Partition FunctionThe probability of the canonical distribution (3.2) is not normalized. Let usnow normalize it. We number the microscopic states of system I, and writethe energy of the ith state as E i . It should be noted that it is possible thatdifferent microscopic states may have the same energy E i .Wefirstcalculatethe following sum Z over all microscopic states:Z(T,V,N)= ∑ (exp − E )i. (3.6)kiB TThe normalized probability is thenf (E i )= e−Ei/kBT. (3.7)ZThe normalization factor Z introduced here is called the partition function.It is a function of the temperature, the volume, and the number ofmolecules, and plays an important role, as we shall see in the next section.However, before discussing its importance, we shall rewrite the above equationin a more convenient form. For a macroscopic system, there are infinitelymany microscopic states. Therefore, it is impossible to carry out the summationin (3.6). We need to calculate the sum by replacing the summationwith an integral. There are many microscopic states between energies E andE +dE. The number of states in this range is given by Ω I (E)dE, whereΩ I (E) is the density of states. On the other hand, for all the states with energyE i in this range, we can write exp(−E i /k B T )asexp(−E/k B T ). The









![Práctica [PDF] - Universidad de Carabobo, FACYT - computacion](https://img.yumpu.com/48491415/1/190x245/practica-pdf-universidad-de-carabobo-facyt-computacion.jpg?quality=85)