Statistical Physics
Statistical Physics
Statistical Physics
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
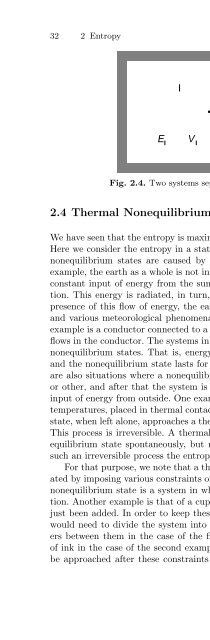
32 2 EntropyFig. 2.4. Two systems separated by a movable wall2.4 Thermal Nonequilibrium and Irreversible ProcessesWe have seen that the entropy is maximized in a state of thermal equilibrium.Here we consider the entropy in a state of thermal nonequilibrium. Thermalnonequilibriumstates are caused by actions from outside the system. Forexample, the earth as a whole is not in thermal equilibrium. There is a nearlyconstant input of energy from the sun in the form of electromagnetic radiation.This energy is radiated, in turn, from the earth to the cosmos. In thepresence of this flow of energy, the earth cannot be in an equilibrium state,and various meteorological phenomena occur and life is supported. Anotherexample is a conductor connected to a battery. In this case an electric currentflows in the conductor. The systems in these examples are in quasi-stationarynonequilibrium states. That is, energy is continuously put into the system,and the nonequilibrium state lasts for a long time. On the other hand, thereare also situations where a nonequilibrium state is prepared by some meansor other, and after that the system is left to evolve by itself without furtherinput of energy from outside. One example is that of two systems at differenttemperatures, placed in thermal contact at some time. Such a nonequilibriumstate, when left alone, approaches a thermal-equilibrium state as time elapses.This process is irreversible. A thermal-nonequilibrium state changes into anequilibrium state spontaneously, but not vice versa. We shall argue that insuch an irreversible process the entropy always increases.For that purpose, we note that a thermal-nonequilibrium state can be createdby imposing various constraints on the system. One example of a typicalnonequilibrium state is a system in which the temperature depends on position.Another example is that of a cup of water to which a droplet of ink hasjust been added. In order to keep these systems in their initial condition, wewould need to divide the system into many small cells with adiabatic barriersbetween them in the case of the first example, and to wrap the dropletof ink in the case of the second example. A thermal-equilibrium state wouldbe approached after these constraints were removed. The molecules acquire









![Práctica [PDF] - Universidad de Carabobo, FACYT - computacion](https://img.yumpu.com/48491415/1/190x245/practica-pdf-universidad-de-carabobo-facyt-computacion.jpg?quality=85)