Statistical Physics
Statistical Physics
Statistical Physics
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
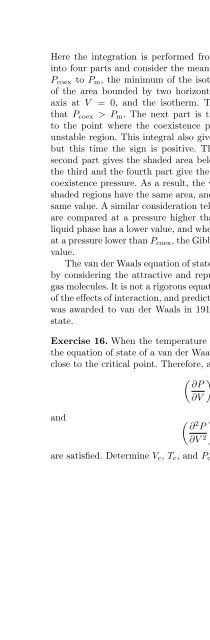
8.6 The van der Waals Gas 131Here the integration is performed from V l to V g . We separate this integralinto four parts and consider the meaning of each part. The first part is fromP coex to P m , the minimum of the isotherm. This integral gives the negativeof the area bounded by two horizontal lines at P coex and P m ,theverticalaxis at V = 0, and the isotherm. The negative sign arises from the factthat P coex >P m . The next part is the integral from P m to P coex , namelyto the point where the coexistence pressure intersects the isotherm in theunstable region. This integral also gives the area to the left of the isotherm,but this time the sign is positive. Therefore, the sum of the first and thesecond part gives the shaded area below the coexistence pressure. Similarly,the third and the fourth part give the negative of the shaded area above thecoexistence pressure. As a result, the whole integral gives zero when the twoshaded regions have the same area, and then the Gibbs free energies have thesame value. A similar consideration tells us that when the Gibbs free energiesare compared at a pressure higher than P coex , the Gibbs free energy of theliquid phase has a lower value, and when the Gibbs free energies are comparedat a pressure lower than P coex , the Gibbs free energy in the gas phase has lowervalue.The van der Waals equation of state was written down phenomenologicallyby considering the attractive and repulsive parts of the interaction betweengas molecules. It is not a rigorous equation of state, but it captures the essenceof the effects of interaction, and predicts a gas–liquid transition. A Nobel Prizewas awarded to van der Waals in 1910 for the discovery of this equation ofstate.Exercise 16. When the temperature is fixed at the critical temperature T c ,the equation of state of a van der Waals gas behaves as P − P c ∝−(V − V c ) 3close to the critical point. Therefore, at (T c ,V c ),( ) ∂P= 0 (8.21)∂Vand( ∂ 2 PT∂V 2 )T= 0 (8.22)are satisfied. Determine V c , T c ,andP c from these equations.









![Práctica [PDF] - Universidad de Carabobo, FACYT - computacion](https://img.yumpu.com/48491415/1/190x245/practica-pdf-universidad-de-carabobo-facyt-computacion.jpg?quality=85)