Introduction to d-Block Chemistry - Wits Structural Chemistry
Introduction to d-Block Chemistry - Wits Structural Chemistry
Introduction to d-Block Chemistry - Wits Structural Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
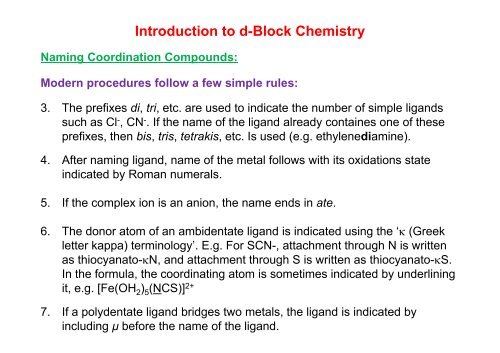
<strong>Introduction</strong> <strong>to</strong> d-<strong>Block</strong> <strong>Chemistry</strong>Naming Coordination Compounds:Modern procedures follow a few simple rules:3. The prefixes di, tri, etc. are used <strong>to</strong> indicate the number of simple ligandssuch as Cl - , CN - . If the name of the ligand already containes one of theseprefixes, then bis, tris, tetrakis, etc. Isused(eg (e.g. ethylenediamine).4. After naming ligand, name of the metal follows with its oxidations stateindicated by Roman numerals.5. If the complex ion is an anion, the name ends in ate.6. The donor a<strong>to</strong>m of an ambidentate ligand is indicated using the ‘κ (Greekletter kappa) terminology’. E.g. For SCN-, attachment through N is writtenas thiocyana<strong>to</strong>-κN, t and attachment t through h S is written as thiocyana<strong>to</strong>-κS. t In the formula, the coordinating a<strong>to</strong>m is sometimes indicated by underliningit, e.g. [Fe(OH 2 ) 5 (NCS)] 2+7. If a polydentate ligand bridges two metals, the ligand is indicated byincluding μ before the name of the ligand.