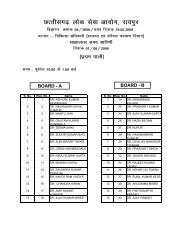
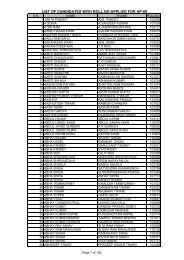
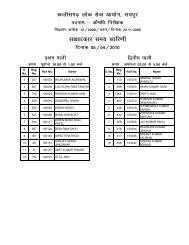
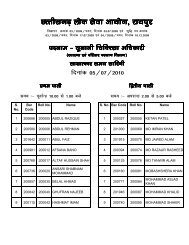
to view / print advertisement (from 28/12/2011) - Chhattisgarh Public ...
to view / print advertisement (from 28/12/2011) - Chhattisgarh Public ...
to view / print advertisement (from 28/12/2011) - Chhattisgarh Public ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
vEy] Fkk;ksfud vEy] Dyksjhu dk foyxu]vfØ;k'khy xSlksa ds ;kSfxd] vUrjrk gSykstu;kSfxd rFkk vkHkklh gSykstu-¼2½ fuEufyf[kr dk fojpu] xq.k/keZ] mi;ksxrFkk ljapuk Mkbcksju] gkbMªksthu] gkbMªksfDly],sehu] gkbiksukbVªsl vEy] lksfM;e Fkk;kslYQsVrFkk gkbMªkstu iSjkDlkbM¼3½ lfØ; ukbVªkstu] ukbVªkbM~l rFkk moZjdksadk foLr`r v/;;u-¼4½ fuEufyf[kr dk fojpu] xq.k/keZ] rFkkmi;ksx & gkbMªkstksbd vEy] VkjVkj,esfVd]ekbØksdkfLed yo.k] Fkk;ksfuy DyksjkbM]lYQ;wfjd DyksjkbM] iksVsf'k;e MkbØksesV]iksVsf'k;e ijeSxusV] Økse,ye] Cyhfpax] ikoMj]iksVsf'k;e QSjks rFkk QSjh lk;ukbM] DyksjkslYQksfud]vEy lksfM;e ckbZ LewFksaV] lksfM;e dksckfYVukbVªkbV],.M dkcksZj.Meiz'u i= f}rh;HkkSfrd dkcZfud jlk;u& bysDVªkfudfoLFkkiu] izsjf.kd] ,ysDVªksesfjd] eSlksesfjd rFkkvfr la;qXeu izHkko] bysDVªksQkby ukfHkdLusgh rFkkeqäewyd] vuqukn rFkk bldk dkcZfud ;kSfxd dsfy;s vuqiz;ksx] dkcZfud vEyksa rFkk {kkjdksa dsfo;kstu fLFkjkad ij lajpuk dk izHkko] gkbMªkstuvkca/k rFkk dkcZfud ;kSfxdksa ds xq.k/keZ ij mldkizHkko -dkcZfud vfHkfØ;kvksa dh fØ;kfof/k dhvk/kqfud /kkj.kk,a] ;ksx'khy fØ;k] izfrLFkkiu fØ;k]foyksiu fØ;k ,oa iqufoZU;kl fØ;k] ,jksesfVdizfrLFkkiuk dh eqä ewyd fØ;k fof/k;ksa esa lekfo"VvfHkfØ;k -csUthu ds e/;orhZ ;kSfxd -,sfyQSfVd jlk;u foKku & fuEufyf[kroxZ ds ljy dkcZfud ;kSfxdksa dk jklk;fudv/;;u ,Ydsu ,Ydhu] ,Ydkbu] ,fYdy gSykbM]vYdksgy] Fkkbvksy ,fYMgkbM] dhVksu ¼,flM½rFkk mlds O;qRiUu bZFkj] ,ehu] ,sehuks vEy]gkbMªkDlh vEy vlar`Ir vEy] f}{kkjdh vEyfuEu;kSfxdksa ds la'ys"k.kkRed mi;ksx &,slhVks,eksfVd rFkk esyksfud ,LVj] esxzsf'k;e rFkkfyfFk;e ds dkcZ/kkfRod ;kSfxd ,tksesFksudkcksZgkbMªsV~l]oxhZdj.k] la:i.k] lkekU;vfHkfØ;k,a ljy eksukslSdjkbM ds lkekU;vfHkfØ;k,a Xyqdkst QDVªkst] lwØkst dk jlk;uf=foejlk;u & lefefr rFkk ljylfefefr lafØ;kvksa ds rRo- ljy dkcZfud v.kqvksaesa izdk'kdh; rFkk T;kferh; leko;rk bZ-tsMrFkkvkj-,l- vadu ljy dkcZfud v.kqvksa dkla:i.k] vdkcZfud leUo;h ;kSfxdksa dk f=foejlk;u],sjkseSfVd jlk;u- csUthu VkWywbZu rFkk mudsgSykstsu] gkbMªkDlh] ukbVªks] rFkk ,ehuksa O;qRiUu]lYQksfjd vEy] tkbfyu csUtykfMgkbM lSfyfly,syfMgkbM] ,slhVksQhuksu] csUtksbd] FkSfyd-(8)lSfyflfyd] flusfedrFkk eSUMsfyd vEy]ukbVªkscsUthu dk vip;u mRikn] Mk;kstksfu;eyo.k rFkk mudk la'ys"k.k esa iz;ksx -QSuuFkzhu] ik;jhMhu rFkk D;wuksyhu uSiFkyhudh lajpuk] la'ys"k.k rFkk egRoiw.kZ vfHkfØ;k-,tks VªkbQsfuy eSFksu rFkk FkSyhu lewgksa dslacaf/kr jatd jax rFkk lajpuk dk vk/kqfud fl)kUrfudksfVu jlk;u ls lacaf/kr lkekU; /kkjk,avkfFkZdrFkk vkS"k/kh; egRo ds &fuEufyf[kr nzO;ksa ls lacaf/kr ladYiuk] lsywykslrFkk LVkpZ] dksyrkj] jlk;fud ;kSfxd] rsy rFkkpchZfuEufyf[krrRoksa dk jlk;u rFkk mudseq[; ;kSfxdksa cksjku] VkbVsfu;e] tesZfu;e]flfydku] fudy] IysfVue] Øksfe;e rFkk ;wjsfu;egkbMªkstu vcU/ku dh izkjafHkd /kkj.kk ch-,l-bZvkj-fl)kUr rFkk vk;fud Bksl inkFkksZ dh ,dkadh;qxylajpuk dk egRo] vk;uu fo'o ds lanHkZ esaNaCl fØLVy tkyd ds fuekZ.k dk ÅtkZ foKkubysDVªku ca/kqrk] rFkk tkyd ÅtkZ ckuZ gkoy pØ]/kzqo.k 'kfä] /kzqo.krk] Qktu dk fu;evEy{kkjd ladYiuk- yksjhØUlVsy ladYiuk]ysfcu ladYiuk] vEy rFkk {kkjdksa ds vU; fl)kUr]dBksj rFkk ueZ vEy {kkjd ladYiuk ih-,p- rFkkmldk fu/kkZj.k] cQj foy;u rFkk cQj fØ;k]gSUMjlu lehdj.k -ek=kRed fo'ys"k.k esa =qfV;ka] =qfV;ksa dkoxhZdj.k =qfV;ksa dk U;wurehdj.k] ifj'kq)rk rFkk;FkkFkZrk] vkadM+ksa dh lkFkZdrk] lg vo{ksi.k rFkkvi{ksi.k] vo{ksi.kksa ds fy;s vuqdwyre n'kk] vEy{kkjd vuqekiuksa esa lwpdksa dk p;u] lwpdksa dsfl)kUr bZMhVh, vuqekiu ds fu;e &¼v½ ,usfyfVdy la[;kRed iz'u ikbjksyqlkbVvk;ksMhfefr] jtr flôs] vEy {kkjd vuqekiu]jsMkd vuqekiu ls lacaf/kr¼c½ ,fyQSfVd rFkk ,jkseSfVd ;kSfxd lslacaf/kr la[;kRed iz'u04 CHEMISTRY :PAPER - I(1) Symmetry :Symmetry, Symmetry elements, Symmetryoprations (general treatments only).(2) Electromagnetic radiations :Electromagnetic radiation, interaction ofelectromagnetic radiations with-matter,consequence of interaction and classification ofelectromagnetic rediation in different regions,Singlet and triplet states . UV and IR spectra andtheir simple applications.(3) A<strong>to</strong>mic structure and chemicalBonding :Sommerfelds model, Schrodinger equation(General treatment) Elementry idea of valencybond and Molecular orbital theory (bonding , nonbondingand antibonding orbitals) diplole momentand Molecular structure.(4) Electro Chemistry.Reversible and irreversible electrodes,Nernst equation, Standard hydrogen electrodeand glass electrode, concentration cells with andwithout transference, condutivity of solution(specific, , equipment and molecular conductance),ionic velocity and effect of dilution onconductance, conduc<strong>to</strong>metric titrations, Degreeof ionisation and conductivity concept of transportnumber and its determination, Kohlrausch's lawof ionic mobility and its applications. Elementaryidea of theory of strong electrolytes.(5) Thermodynamics :Extensive and intensive properties, isolatedsystem. Intrinsic energy, and enthalpy asproperties of states, Reversible , irreversible andcyclic processes and drive <strong>to</strong>wards equillibrium,Second law of the thermodynamics with reference<strong>to</strong> entropy, physical concept of entropy, simpleapplications of thermodyanmics <strong>to</strong> chemicalprocesses.(6) Nuclear Chemistry :Theory of a<strong>to</strong>mic nucleus and nuclearstability in terms of N/P ratio, binding enegry,packing fraction, mass defect, whole number rule,Effective nuclear charge, a<strong>to</strong>mic disintegration,nuclear section, nuclear cross-section, basicprinciples of nuclear fission and fusion.(7) Chemistry of elements and theircompounds:A comparative study of XIV, XV, XVI,XVII and zero group elements with respect <strong>to</strong>electronic configuration,oxidation states ,hydrides, oxides , halides and hydroxides.(8) Transition and inner transitionelements :Electronic configuration, position in theperiodic table, characteristics, oxidation states.spectral and magnetic properties of transitionelements.General study, electronic structure,oxidation states, spectral and magnetic propertiesof lanthanides, lanthanide contraction.(9) Co-ordination Chemistry :Werner's theory of co-ordinationcompounds, its electronic interpretation valencybond theory for explaining metalling and bonding,hybridisation in complexes.(10) Non-aqueous solvents :Classification and characteristics ofsolvents Chemistry of non-aqueous solvents withspecial reference <strong>to</strong> liquid ammonia and liquidsulphur dioxide.(11) Inorganic Compounds :(i) A detailed study of oxides. oxyacids(including electronic structure ) of nitrogen,phosphorus, and sulphur. Peroxoacids of sulphur.Øe'k%