106 S. P. R<strong>in</strong>ger <strong>and</strong> K. Honoelements of the phase are common tothose of the m3m -matrix, the <strong>in</strong>tersectionpo<strong>in</strong>t group is the same as that of phase:4/mmm. Because the po<strong>in</strong>t group of thematrix phase conta<strong>in</strong>s 48 symmetry elements<strong>and</strong> the <strong>in</strong>tersection po<strong>in</strong>t groupconta<strong>in</strong>s 16, the <strong>in</strong>dex of the <strong>in</strong>tersectionpo<strong>in</strong>t group <strong>in</strong> the -matrix is 48/16 3.Symmetry thus requires three crystallographicallyequivalent variants. The specialcrystal forms compatible with this po<strong>in</strong>tgroup [33] <strong>in</strong>clude p<strong>in</strong>acoids normal to thefourfold axis, <strong>and</strong> tetragonal prisms parallelto twofold axes possess<strong>in</strong>g the requiredmirror symmetry, which is consistent withobservation.The tetragonal phase (I4/mcm, a 0.6066nm, c 0.4874nm) [34] occurs <strong>in</strong> avariety of orientations <strong>and</strong> morphologies.These have been systematically exam<strong>in</strong>edby Vaughan <strong>and</strong> Silcock, who showed thatthere are 159 known orientations for a particle relative to the -Al matrix [35].EFFECTS OF Sn, Cd, AND In ONPRECIPITATION IN Al–Cu ALLOYSTrace additions of Cd, In, <strong>and</strong> Sn <strong>in</strong>creaseboth the rate <strong>and</strong> extent of harden<strong>in</strong>g <strong>in</strong> Al–Cu alloys aged at temperatures between100 to 200C [36–38] (Fig. 2). Comparisonsbetween b<strong>in</strong>ary <strong>and</strong> ternary alloys haveshown that, whereas these elements suppressthe formation of the GP zones <strong>and</strong> the phase, they stimulate a f<strong>in</strong>er <strong>and</strong> moreuniform dispersion of the semicoherent .Two types of proposals have been offeredto describe the mechanisms for this ref<strong>in</strong>edprecipitate dispersion. One hypothesis isthat the trace elements are absorbed at the/matrix <strong>in</strong>terfaces, result<strong>in</strong>g <strong>in</strong> a reductionof the <strong>in</strong>terfacial energy required forprecipitate nucleation. This explanationwas first proposed by Silcock et al. [37] toaccount for weak X-ray reflections (designated“p-diffractions”) observed dur<strong>in</strong>gthe early stages of ag<strong>in</strong>g. This proposal received<strong>in</strong>direct experimental support fromcalorimetric measurements by Boyd <strong>and</strong>Nicholson [39] <strong>and</strong> TEM observations bySankaren <strong>and</strong> Laird [40], who claimed todetect what they referred to as Sn (Cd or In)segregates <strong>and</strong> precipitates <strong>in</strong> associationwith the particle/matrix <strong>in</strong>terface. An alternativeexplanation is that the trace elementsfacilitate heterogeneous nucleationof either directly at Sn (Cd or In)-richparticles [41–43], or <strong>in</strong>directly at the dislocationloops present <strong>in</strong> the AQ microstructure[44].R<strong>and</strong>om area 1DAP analyses obta<strong>in</strong>edfrom samples of an Al–1.7Cu–0.01Sn alloy<strong>in</strong>dicate that the Sn atoms are not homogeneouslydistributed <strong>in</strong> the -matrix immediatelyafter quench<strong>in</strong>g from the solutiontreatment temperature [45]. Rather, theyoccur <strong>in</strong> discrete clusters, <strong>and</strong> there was noevidence of enrichment of Cu at their locations.As is usual <strong>in</strong> the exam<strong>in</strong>ation of AQAl–Cu-based alloys, occasional clusters ofCu atoms were also observed, but thesewere not spatially correlated with the Snclusters. A statistical test of the atom-probedata, known as cont<strong>in</strong>gency table analysis,also <strong>in</strong>dicated that Cu <strong>and</strong> Sn did not havea preferential <strong>in</strong>teraction <strong>in</strong> the cluster<strong>in</strong>gstage.Figure 4(a) is an He FIM image of the Al–1.7Cu–0.01Sn alloy aged 3 m<strong>in</strong> at 190C <strong>and</strong>shows brightly imag<strong>in</strong>g precipitates, <strong>in</strong>dicatedby arrows. The selected area 1DAPanalysis presented <strong>in</strong> Fig. 4(b) was obta<strong>in</strong>edby prob<strong>in</strong>g at or near these brightly imag<strong>in</strong>gregions, which were found to be Curich nuclei, heterogeneously nucleatedon Sn particles. Figure 4(b) is an example ofan analysis through the /Sn <strong>in</strong>terface.The Cu ladder is uniform through both theSn particle <strong>and</strong> the adjacent matrix, confirm<strong>in</strong>gthat there is no Cu enrichment <strong>in</strong>the particles. However, there is a sharp <strong>in</strong>terfacebetween the Sn-rich <strong>and</strong> Cu-richprecipitates. Note that <strong>in</strong> Fig. 4(b), the compositionof the Cu-rich precipitate is 33 at.% Cu, which corresponds to the nom<strong>in</strong>alcomposition of (Al 2 Cu). This observation<strong>and</strong> the fact that Al <strong>and</strong> Sn have extremelylimited solid solubility <strong>in</strong> each other suggestthat the particles are pure Sn. Microbeamelectron diffraction confirmed thepresence of the -Sn phase (I4 1 /amd, a 0.583nm, c 0.318nm) oriented such that
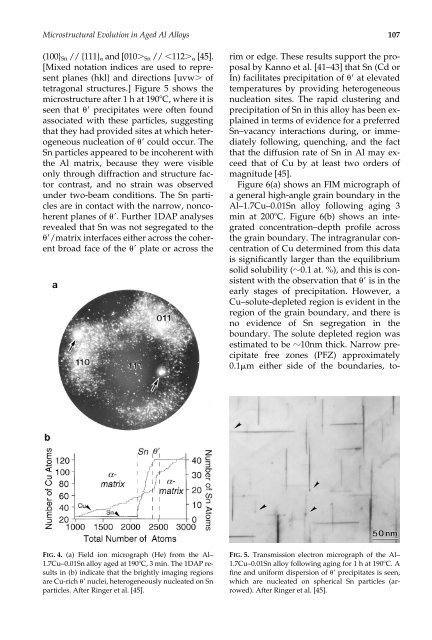
<strong>Microstructural</strong> <strong>Evolution</strong> <strong>in</strong> <strong>Age</strong>d Al <strong>Alloys</strong> 107(100} Sn // {111} <strong>and</strong> [010 Sn // 112 [45].[Mixed notation <strong>in</strong>dices are used to representplanes (hkl} <strong>and</strong> directions [uvw oftetragonal structures.] Figure 5 shows themicrostructure after 1 h at 190C, where it isseen that precipitates were often foundassociated with these particles, suggest<strong>in</strong>gthat they had provided sites at which heterogeneousnucleation of could occur. TheSn particles appeared to be <strong>in</strong>coherent withthe Al matrix, because they were visibleonly through diffraction <strong>and</strong> structure factorcontrast, <strong>and</strong> no stra<strong>in</strong> was observedunder two-beam conditions. The Sn particlesare <strong>in</strong> contact with the narrow, noncoherentplanes of . Further 1DAP analysesrevealed that Sn was not segregated to the/matrix <strong>in</strong>terfaces either across the coherentbroad face of the plate or across therim or edge. These results support the proposalby Kanno et al. [41–43] that Sn (Cd orIn) facilitates precipitation of at elevatedtemperatures by provid<strong>in</strong>g heterogeneousnucleation sites. The rapid cluster<strong>in</strong>g <strong>and</strong>precipitation of Sn <strong>in</strong> this alloy has been expla<strong>in</strong>ed<strong>in</strong> terms of evidence for a preferredSn–vacancy <strong>in</strong>teractions dur<strong>in</strong>g, or immediatelyfollow<strong>in</strong>g, quench<strong>in</strong>g, <strong>and</strong> the factthat the diffusion rate of Sn <strong>in</strong> Al may exceedthat of Cu by at least two orders ofmagnitude [45].Figure 6(a) shows an FIM micrograph ofa general high-angle gra<strong>in</strong> boundary <strong>in</strong> theAl–1.7Cu–0.01Sn alloy follow<strong>in</strong>g ag<strong>in</strong>g 3m<strong>in</strong> at 200C. Figure 6(b) shows an <strong>in</strong>tegratedconcentration–depth profile acrossthe gra<strong>in</strong> boundary. The <strong>in</strong>tragranular concentrationof Cu determ<strong>in</strong>ed from this datais significantly larger than the equilibriumsolid solubility (0.1 at. %), <strong>and</strong> this is consistentwith the observation that is <strong>in</strong> theearly stages of precipitation. However, aCu–solute-depleted region is evident <strong>in</strong> theregion of the gra<strong>in</strong> boundary, <strong>and</strong> there isno evidence of Sn segregation <strong>in</strong> theboundary. The solute depleted region wasestimated to be 10nm thick. Narrow precipitatefree zones (PFZ) approximately0.1m either side of the boundaries, to-FIG. 4. (a) Field ion micrograph (He) from the Al–1.7Cu–0.01Sn alloy aged at 190C, 3 m<strong>in</strong>. The 1DAP results<strong>in</strong> (b) <strong>in</strong>dicate that the brightly imag<strong>in</strong>g regionsare Cu-rich nuclei, heterogeneously nucleated on Snparticles. After R<strong>in</strong>ger et al. [45].FIG. 5. Transmission electron micrograph of the Al–1.7Cu–0.01Sn alloy follow<strong>in</strong>g ag<strong>in</strong>g for 1 h at 190C. Af<strong>in</strong>e <strong>and</strong> uniform dispersion of precipitates is seen,which are nucleated on spherical Sn particles (arrowed).After R<strong>in</strong>ger et al. [45].