Palmetto GBA Test Identifier (PTI) Application Guide
Palmetto GBA Test Identifier (PTI) Application Guide
Palmetto GBA Test Identifier (PTI) Application Guide
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
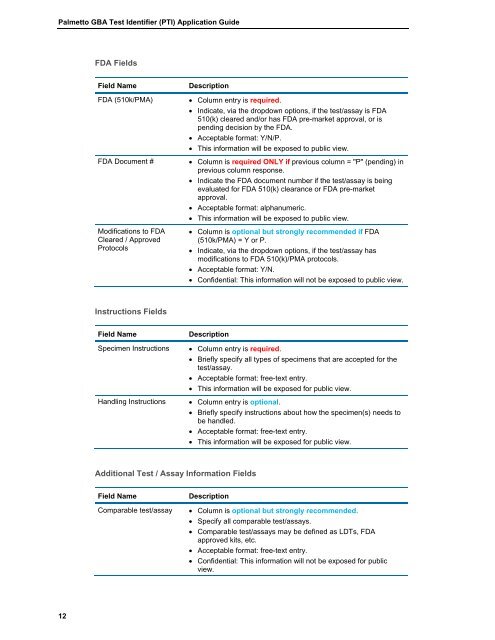
<strong>Palmetto</strong> <strong>GBA</strong> <strong>Test</strong> <strong>Identifier</strong> (<strong>PTI</strong>) <strong>Application</strong> <strong>Guide</strong>FDA FieldsField NameFDA (510k/PMA)FDA Document #Modifications to FDACleared / ApprovedProtocolsDescription Column entry is required. Indicate, via the dropdown options, if the test/assay is FDA510(k) cleared and/or has FDA pre-market approval, or ispending decision by the FDA. Acceptable format: Y/N/P. This information will be exposed to public view. Column is required ONLY if previous column = "P" (pending) inprevious column response. Indicate the FDA document number if the test/assay is beingevaluated for FDA 510(k) clearance or FDA pre-marketapproval. Acceptable format: alphanumeric. This information will be exposed to public view. Column is optional but strongly recommended if FDA(510k/PMA) = Y or P. Indicate, via the dropdown options, if the test/assay hasmodifications to FDA 510(k)/PMA protocols. Acceptable format: Y/N. Confidential: This information will not be exposed to public view.Instructions FieldsField NameSpecimen InstructionsHandling InstructionsDescription Column entry is required. Briefly specify all types of specimens that are accepted for thetest/assay. Acceptable format: free-text entry. This information will be exposed for public view. Column entry is optional. Briefly specify instructions about how the specimen(s) needs tobe handled. Acceptable format: free-text entry. This information will be exposed for public view.Additional <strong>Test</strong> / Assay Information FieldsField NameComparable test/assayDescription Column is optional but strongly recommended. Specify all comparable test/assays. Comparable test/assays may be defined as LDTs, FDAapproved kits, etc. Acceptable format: free-text entry. Confidential: This information will not be exposed for publicview.12