An Exercise in Freezing Point Depression
An Exercise in Freezing Point Depression
An Exercise in Freezing Point Depression
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
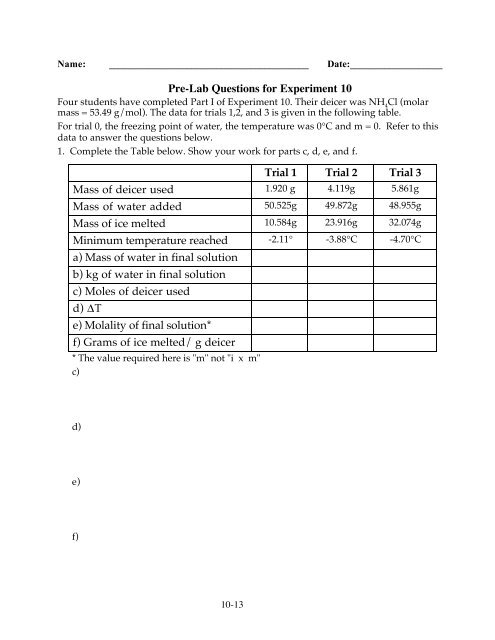
Name: _________________________________________ Date:___________________Pre-Lab Questions for Experiment 10Four students have completed Part I of Experiment 10. Their deicer was NH 4Cl (molarmass = 53.49 g/mol). The data for trials 1,2, and 3 is given <strong>in</strong> the follow<strong>in</strong>g table.For trial 0, the freez<strong>in</strong>g po<strong>in</strong>t of water, the temperature was 0°C and m = 0. Refer to thisdata to answer the questions below.1. Complete the Table below. Show your work for parts c, d, e, and f.Trial 1 Trial 2 Trial 3Mass of deicer used 1.920 g 4.119g 5.861gMass of water added 50.525g 49.872g 48.955gMass of ice melted 10.584g 23.916g 32.074gM<strong>in</strong>imum temperature reached -2.11° -3.88°C -4.70°Ca) Mass of water <strong>in</strong> f<strong>in</strong>al solutionb) kg of water <strong>in</strong> f<strong>in</strong>al solutionc) Moles of deicer usedd) ∆Te) Molality of f<strong>in</strong>al solution*f) Grams of ice melted/ g deicer* The value required here is "m" not "i x m"c)d)e)f)10-13