Pro-oxidant activity of vitamin C in drinking water ... - Ã bo Akademi
Pro-oxidant activity of vitamin C in drinking water ... - Ã bo Akademi
Pro-oxidant activity of vitamin C in drinking water ... - Ã bo Akademi
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
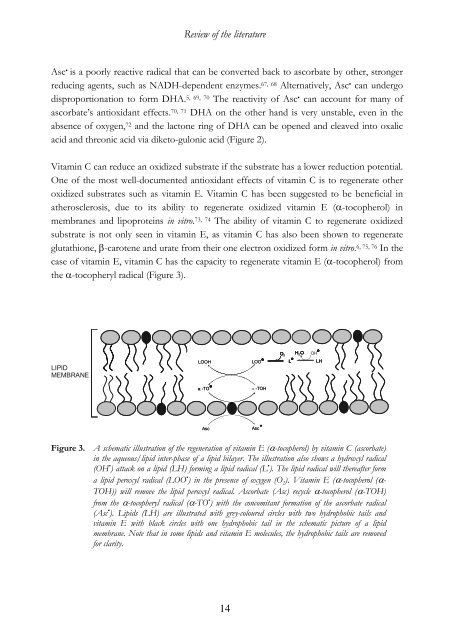
Review <strong>of</strong> the literatureAsc • is a poorly reactive radical that can be converted back to ascorbate by other, strongerreduc<strong>in</strong>g agents, such as NADH-dependent enzymes. 67, 68 Alternatively, Asc • can undergodisproportionation to form DHA. 5, 69, 70 The re<strong>activity</strong> <strong>of</strong> Asc • can account for many <strong>of</strong>ascorbate’s anti<strong>oxidant</strong> effects. 70, 71 DHA on the other hand is very unstable, even <strong>in</strong> theabsence <strong>of</strong> oxygen, 72 and the lactone r<strong>in</strong>g <strong>of</strong> DHA can be opened and cleaved <strong>in</strong>to oxalicacid and threonic acid via diketo-gulonic acid (Figure 2).Vitam<strong>in</strong> C can reduce an oxidized substrate if the substrate has a lower reduction potential.One <strong>of</strong> the most well-documented anti<strong>oxidant</strong> effects <strong>of</strong> <strong>vitam<strong>in</strong></strong> C is to regenerate otheroxidized substrates such as <strong>vitam<strong>in</strong></strong> E. Vitam<strong>in</strong> C has been suggested to be beneficial <strong>in</strong>atherosclerosis, due to its ability to regenerate oxidized <strong>vitam<strong>in</strong></strong> E (α-tocopherol) <strong>in</strong>membranes and lipoprote<strong>in</strong>s <strong>in</strong> vitro. 73, 74 The ability <strong>of</strong> <strong>vitam<strong>in</strong></strong> C to regenerate oxidizedsubstrate is not only seen <strong>in</strong> <strong>vitam<strong>in</strong></strong> E, as <strong>vitam<strong>in</strong></strong> C has also been shown to regenerateglutathione, β-carotene and urate from their one electron oxidized form <strong>in</strong> vitro. 6, 75, 76 In thecase <strong>of</strong> <strong>vitam<strong>in</strong></strong> E, <strong>vitam<strong>in</strong></strong> C has the capacity to regenerate <strong>vitam<strong>in</strong></strong> E (α-tocopherol) fromthe α-tocopheryl radical (Figure 3).O 2HO2 OHLLHLIPIDMEMBRANELOOHLOOα-TOα-TOHAscAscFigure 3. A schematic illustration <strong>of</strong> the regeneration <strong>of</strong> <strong>vitam<strong>in</strong></strong> E (α-tocopherol) by <strong>vitam<strong>in</strong></strong> C (ascorbate)<strong>in</strong> the aqueous/lipid <strong>in</strong>ter-phase <strong>of</strong> a lipid bilayer. The illustration also shows a hydroxyl radical(OH • ) attack on a lipid (LH) form<strong>in</strong>g a lipid radical (L • ). The lipid radical will thereafter forma lipid peroxyl radical (LOO • ) <strong>in</strong> the presence <strong>of</strong> oxygen (O 2 ). Vitam<strong>in</strong> E (α-tocopherol (α-TOH)) will remove the lipid peroxyl radical. Ascorbate (Asc) recycle α-tocopherol (α-TOH)from the α-tocopheryl radical (α-TO • ) with the concomitant formation <strong>of</strong> the ascorbate radical(Asc • ). Lipids (LH) are illustrated with grey-coloured circles with two hydrophobic tails and<strong>vitam<strong>in</strong></strong> E with black circles with one hydrophobic tail <strong>in</strong> the schematic picture <strong>of</strong> a lipidmembrane. Note that <strong>in</strong> some lipids and <strong>vitam<strong>in</strong></strong> E molecules, the hydrophobic tails are removedfor clarity.14