Zinc-EDTA Complexation Reaction
Zinc-EDTA Complexation Reaction
Zinc-EDTA Complexation Reaction
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Zinc</strong> HydroxideZn 2+ + 2OH - → Zn(OH)2(s)Solubility productKsp = 3.0×10 −17Ksp = [Zn 2+ ][OH - ] 2At pH=10, [Zn 2+ ] = Ksp /[OH - ] 2
Ammonia Buffer SolutionNH3 + H2O NH4 + + OH - pKb = 4.74[NH3]total = 0.100MAt pH=10, [NH3] = ??
<strong>Zinc</strong> - Ammonia <strong>Complexation</strong>At [NH3] = 0.0846M, αZn2+ = 1.61 x 10 -5
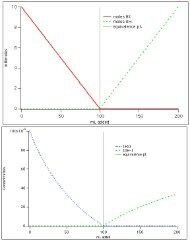
<strong>EDTA</strong> TitrationYou would like to perform a titration of 50.00 mL of a1.00 x 10 -4 M Zn 2+ solution with a 1.00 x 10 -4 M <strong>EDTA</strong>solution.Total moles of <strong>Zinc</strong> = ??
<strong>EDTA</strong> TitrationYou would like to perform a titration of 50.00 mL of a1.00 x 10 -4 M Zn 2+ solution with a 1.00 x 10 -4 M <strong>EDTA</strong>solution.Total moles of <strong>Zinc</strong> = (1.00 x 10 -4 M)(0.050L)= 5.0 x 10 -6 moles
<strong>EDTA</strong> TitrationYou would like to perform a titration of 50.00 mL of a1.00 x 10 -4 M Zn 2+ solution with a 1.00 x 10 -4 M <strong>EDTA</strong>solution.Total moles of <strong>Zinc</strong> = 5.0 x 10 -6 molesEquivalence point volume = ??
<strong>EDTA</strong> TitrationYou would like to perform a titration of 50.00 mL of a1.00 x 10 -4 M Zn 2+ solution with a 1.00 x 10 -4 M <strong>EDTA</strong>solution.Total moles of <strong>Zinc</strong> = 5.0 x 10 -6 molesEquivalence point volume = 0.100L
<strong>EDTA</strong> TitrationYou would like to perform a titration of 50.00 mL of a1.00 x 10 -4 M Zn 2+ solution with a 1.00 x 10 -4 M <strong>EDTA</strong>solution.Total moles of <strong>Zinc</strong> = 5.0 x 10 -6 molesEquivalence point volume = 0.100L[ ZnY 2- ] = ??
<strong>EDTA</strong> TitrationYou would like to perform a titration of 50.00 mL of a1.00 x 10 -4 M Zn 2+ solution with a 1.00 x 10 -4 M <strong>EDTA</strong>solution.Total moles of <strong>Zinc</strong> = 5.0 x 10 -6 molesEquivalence point volume = 0.100L[ ZnY 2- ] = 5.0 x 10 -5 MWe assume a stoichiometric reaction.
<strong>EDTA</strong> TitrationYou would like to perform a titration of 50.00 mL of a1.00 x 10 -4 M Zn 2+ solution with a 1.00 x 10 -4 M <strong>EDTA</strong>solution.Total moles of <strong>Zinc</strong> = 5.0 x 10 -6 molesEquivalence point volume = 0.100L[ ZnY 2- ] = 5.0 x 10 -5 M[ Zn 2+ ] = ??
[ ZnY 2- ] = 5.0 x 10 -5 MWe assumed a stoichiometric reaction. But actually, thereis a little bit of free (uncomplexed) <strong>EDTA</strong> and free(uncomplexed) <strong>Zinc</strong> in solution.
[ ZnY 2- ] = 5.0 x 10 -5 MWe assumed a stoichiometric reaction. But actually, thereis a little bit of free (uncomplexed) <strong>EDTA</strong> and free(uncomplexed) <strong>Zinc</strong> in solution.Log Kf = 16.5.αY4- = 0.355αZn2+ = 1.61 x 10 -5
[ ZnY 2- ] = 5.0 x 10 -5 MWe assumed a stoichiometric reaction. But actually, thereis a little bit of free (uncomplexed) <strong>EDTA</strong> and free(uncomplexed) <strong>Zinc</strong> in solution.2.35 x 10 -6 M
<strong>EDTA</strong> TitrationYou would like to perform a titration of 50.00 mL of a1.00 x 10 -4 M Zn 2+ solution with a 1.00 x 10 -4 M <strong>EDTA</strong>solution.Total moles of zinc = 5.0 x 10 -6 molesEquivalence point volume = 0.100L[ ZnY 2- ] = 5.0 x 10 -5 M 2.35 x 10 -6 M
<strong>EDTA</strong> TitrationYou would like to perform a titration of 50.00 mL of a1.00 x 10 -4 M Zn 2+ solution with a 1.00 x 10 -4 M <strong>EDTA</strong>solution.Total moles of zinc = 5.0 x 10 -6 molesEquivalence point volume = 0.100L[ ZnY 2- ] = 5.0 x 10 -5 M2.35 x 10 -6 M= (1.61 x 10 -5 ) (2.35 x 10 -6 M)[ Zn 2+ ] = 3.78 x 10 -11 M pZn = 10.4We are done!