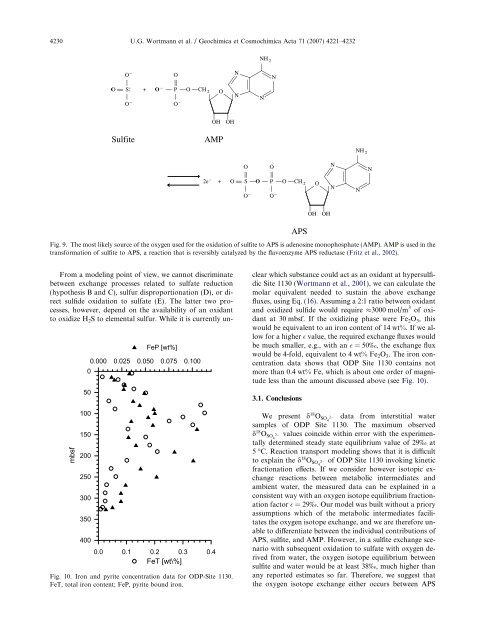
4230 U.G. Wortmann et al. / Geochimica et Cosmochimica Acta 71 (2007) 4221–4232Fig. 9. The most likely source <strong>of</strong> <strong>the</strong> oxygen used for <strong>the</strong> oxidation <strong>of</strong> sulfite to APS is adenos<strong>in</strong>e monophosphate (AMP). AMP is used <strong>in</strong> <strong>the</strong>transformation <strong>of</strong> sulfite to APS, a reaction that is reversibly catalyzed by <strong>the</strong> flavoenzyme APS reductase (Fritz et al., 2002).mbsf050100150200250300350400FeP [wt%]0.000 0.025 0.050 0.075 0.1000.0 0.1 0.2 0.3 0.4FeT [wt\%]Fig. 10. Iron and pyrite concentration data for ODP-Site 1130.FeT, total iron content; FeP, pyrite bound iron.From a model<strong>in</strong>g po<strong>in</strong>t <strong>of</strong> view, we cannot discrim<strong>in</strong>atebetween exchange processes related to <strong>sulfate</strong> reduction(hypo<strong>the</strong>sis B and C), sulfur disproportionation (D), or directsulfide oxidation to <strong>sulfate</strong> (E). The latter two processes,however, depend on <strong>the</strong> availability <strong>of</strong> an oxidantto oxidize H 2 S to elemental sulfur. While it is currently unclearwhich substance could act as an oxidant at hypersulfidicSite 1130 (Wortmann et al., 2001), we can calculate <strong>the</strong>molar equivalent needed to susta<strong>in</strong> <strong>the</strong> above exchangefluxes, us<strong>in</strong>g Eq. (16). Assum<strong>in</strong>g a 2:1 ratio between oxidantand oxidized sulfide would require 3000 mol/m 3 <strong>of</strong> oxidantat 30 mbsf. If <strong>the</strong> oxidiz<strong>in</strong>g phase were Fe 2 O 3 , thiswould be equivalent to an iron content <strong>of</strong> 14 wt%. If we allowfor a higher value, <strong>the</strong> required exchange fluxes wouldbe much smaller, e.g., with an =50‰, <strong>the</strong> exchange fluxwould be 4-fold, equivalent to 4 wt% Fe 2 O 3 . The iron concentrationdata shows that ODP Site 1130 conta<strong>in</strong>s notmore than 0.4 wt% Fe, which is about one order <strong>of</strong> magnitudeless than <strong>the</strong> amount discussed above (see Fig. 10).3.1. ConclusionsWe present d 18 O SO4 2 data from <strong>in</strong>terstitial <strong>water</strong>samples <strong>of</strong> ODP Site 1130. The maximum observedd 18 O SO4 2 values co<strong>in</strong>cide with<strong>in</strong> error with <strong>the</strong> experimentallydeterm<strong>in</strong>ed steady state equilibrium value <strong>of</strong> 29‰ at5 °C. Reaction transport model<strong>in</strong>g shows that it is difficultto expla<strong>in</strong> <strong>the</strong> d 18 O SO4 2 <strong>of</strong> ODP Site 1130 <strong>in</strong>vok<strong>in</strong>g k<strong>in</strong>eticfractionation effects. If we consider however isotopic exchangereactions between metabolic <strong>in</strong>termediates andambient <strong>water</strong>, <strong>the</strong> measured data can be expla<strong>in</strong>ed <strong>in</strong> aconsistent way with an oxygen <strong>isotope</strong> equilibrium fractionationfactor =29‰. Our model was built without a prioryassumptions which <strong>of</strong> <strong>the</strong> metabolic <strong>in</strong>termediates facilitates<strong>the</strong> oxygen <strong>isotope</strong> exchange, and we are <strong>the</strong>refore unableto differentiate between <strong>the</strong> <strong>in</strong>dividual contributions <strong>of</strong>APS, sulfite, and AMP. However, <strong>in</strong> a sulfite exchange scenariowith subsequent oxidation to <strong>sulfate</strong> with oxygen derivedfrom <strong>water</strong>, <strong>the</strong> oxygen <strong>isotope</strong> equilibrium betweensulfite and <strong>water</strong> would be at least 38‰, much higher thanany reported estimates so far. Therefore, we suggest that<strong>the</strong> oxygen <strong>isotope</strong> exchange ei<strong>the</strong>r occurs between APS
<strong>Oxygen</strong> <strong>isotope</strong> <strong>biogeochemistry</strong> <strong>of</strong> <strong>pore</strong> <strong>water</strong> <strong>sulfate</strong>... 4231and <strong>water</strong>, or that sulfite is oxidized to <strong>sulfate</strong> with enrichedoxygen derived from AMP. The latter solution requires that<strong>the</strong> oxygen isotopic sulfite–<strong>water</strong> equilibrium value isaround 30‰, which is close to <strong>the</strong> value implied by o<strong>the</strong>rstudies. We cannot fully discount <strong>the</strong> possibility that <strong>the</strong> exchangereaction occurs with a metabolic <strong>in</strong>termediate <strong>in</strong> <strong>the</strong>oxidative part <strong>of</strong> <strong>the</strong> sulfur cycle, but we show that thiswould require up to 3000 mol/m 3 <strong>of</strong> oxidant, which is<strong>in</strong>consistent with <strong>the</strong> sediment geochemical data.The calculated volumetric oxygen <strong>isotope</strong> exchangefluxes are on average 14 times higher than <strong>the</strong> correspond<strong>in</strong>gSRR. This suggests that only a small fraction <strong>of</strong> <strong>the</strong> <strong>sulfate</strong>enter<strong>in</strong>g <strong>the</strong> cell is used to ga<strong>in</strong> metabolic energy. Theratio between <strong>the</strong> modeled fluxes appears to be depth<strong>in</strong>variant over large <strong>in</strong>tervals, and we hypo<strong>the</strong>size that thisbehavior is caused by decreas<strong>in</strong>g cell densities with depth,while <strong>the</strong> cell-specific SRRs rema<strong>in</strong> constant.ACKNOWLEDGMENTSDiscussions with Laura Lee and James Walker, and <strong>the</strong>comments <strong>of</strong> three anonymous reviewers helped to focus ourideas. M.E.B. thanks S. Lilienthal and <strong>the</strong> Max Planck Societyfor support. U.G.W. thanks <strong>the</strong> Natural Sciences and Eng<strong>in</strong>eer<strong>in</strong>gResearch Council Canada NSERC, which supported thisstudy, and <strong>the</strong> German Science Foundation DFG which supportedhis participation on ODP Leg 182. U.G.W. and P.K.S.thank crew, scientific party, and technicians <strong>of</strong> <strong>the</strong> JOIDESResolution on ODP Leg 182 for <strong>the</strong>ir support and commitmentwhich facilitated <strong>the</strong> recovery <strong>of</strong> <strong>the</strong>se samples under difficultconditions. The authors thank B. Schnetger (ICBM Oldenburg)for XRF analyses and M.E.B. thanks A. Schipper for technicalassistance.REFERENCESAharon P. and Fu B. (2000) Microbial <strong>sulfate</strong> reduction rates andsulfur oxygen <strong>isotope</strong> fractionations at oil and gas seeps <strong>in</strong><strong>deep</strong><strong>water</strong> Gulf <strong>of</strong> Mexico. Geochim. Cosmochim. Acta 64(2),233–246.Aharon P. and Fu B. (2003) Sulfur and oxygen <strong>isotope</strong>s <strong>of</strong> coeval<strong>sulfate</strong>–sulfide <strong>in</strong> <strong>pore</strong> fluids <strong>of</strong> cold seep sediments with sharpredox gradients. Chem. Geol. 195, 201–218.Berg P., Risgaard-Petersen N. and Rysgaard S. (1998) Interpretation<strong>of</strong> measured concentration pr<strong>of</strong>iles <strong>in</strong> sediment <strong>pore</strong> <strong>water</strong>.Limnol. Oceanogr. 43(7), 1500–1510.Berner R. A. (1964) An idealized model <strong>of</strong> dissolved <strong>sulfate</strong>distribution <strong>in</strong> recent sediments. Geochim. Cosmochim. Acta 28,1497–1503.Berner R. A. (1980) Early Diagenesis: A Theoretical Approach.Pr<strong>in</strong>ceton University Press, Pr<strong>in</strong>ceton, New Jersey.Berner R. A. (1982) Burial <strong>of</strong> organic carbon and pyrite sulfur <strong>in</strong><strong>the</strong> modern ocean: its geochemical and environmental significance.J. Foram. Res. 282, 451–473.Blake, R. E., Surkov, A. V., Böttcher, M. E., Ferdelman, T. G. andJørgensen, B. B. (2006) <strong>Oxygen</strong> <strong>isotope</strong> composition <strong>of</strong>dissolved <strong>sulfate</strong> <strong>in</strong> <strong>deep</strong>-sea sediments: Eastern EquatorialPacific Ocean. In Proceed<strong>in</strong>gs <strong>of</strong> <strong>the</strong> Ocean Drill<strong>in</strong>g Programm,Scientific Results, vol. 201 (eds. B. B. Jørgensen, S. L. D’Hondtand D. J. Miller). ODP, pp. 1–24.Böttcher M., Hespenheide B., Brumsack H. and Bosselmann K.(2004) Stable <strong>isotope</strong> <strong>biogeochemistry</strong> <strong>of</strong> <strong>the</strong> sulfur cycle <strong>in</strong>modern mar<strong>in</strong>e sediments: I. Seasonal dynamics <strong>in</strong> a temperate<strong>in</strong>tertidal sandy surface sediment. Isotopes Environ. HealthStudies 40, 267–283.Böttcher M., Thamdrup B. and Vennemann T. W. (2001) <strong>Oxygen</strong>and sulfur <strong>isotope</strong> fractionation dur<strong>in</strong>g anaerobic bacterialdisproportionation <strong>of</strong> elemental sulfur. Geochim. Cosmochim.Acta 65, 1601–1609.Böttcher M. E., Thamdrup B., Gehre M. and Theune A. (2005)34 S/ 32 S and 18 O/ 16 O fractionation dur<strong>in</strong>g sulfur disproportionationby Desulfobulbus propionicus. Geomicrobiol. J. 22,219–226.Böttcher, M. E., Bernasconi, S. M. and Brumsack, H. J. (1999)Carbon and sulfur <strong>isotope</strong> geochemistry <strong>of</strong> <strong>in</strong>terstitial <strong>water</strong>sfrom <strong>the</strong> western Mediterranean. In Proceed<strong>in</strong>gs <strong>of</strong> <strong>the</strong> OceanDrill<strong>in</strong>g Program, Scientific Results, Leg 161 (eds. M. Comas,R. Zahn and A. Klaus), College Station, TX (Ocean Drill<strong>in</strong>gProgram), pp. 413–421.Böttcher, M. E., Brumsack, H. J. and de Lange, G. J. (1998)Sulfate reduction and related stable <strong>isotope</strong> (d 34 S, d 18 O)variations <strong>in</strong> <strong>in</strong>terstitial <strong>water</strong>s <strong>of</strong> <strong>the</strong> eastern Mediterranean.In Proceed<strong>in</strong>gs <strong>of</strong> <strong>the</strong> Ocean Drill<strong>in</strong>g Programm, ScientificResults, vol. 160 (ed. A. H. F. Robertson et al.). CollegeStation, TX (Ocean Drill<strong>in</strong>g Program), pp. 365–373.Bottrell S. H., Parkes R. J., Cragg B. A. and Raiswell R. (2000)Isotopic evidence for anoxic pyrite oxidation and stimulation <strong>of</strong>bacterial sulphate reduction <strong>in</strong> mar<strong>in</strong>e sediments. J. Geol. Soc.157, 711–714.Boudreau B. P. (1996) Diagenetic Models and <strong>the</strong>ir Implementation.Spr<strong>in</strong>ger, New York.Boudreau B. P. and Westrich J. T. (1984) The dependence <strong>of</strong>bacterial <strong>sulfate</strong> reduction on <strong>sulfate</strong> concentration <strong>in</strong> mar<strong>in</strong>esediments. Geochim. Cosmochim. Acta 48, 2503–2516.Brüchert, V. (2004) Physiological and ecological aspects <strong>of</strong> sulfur<strong>isotope</strong> fractionation dur<strong>in</strong>g bacterial <strong>sulfate</strong> reduction. InSulfur Biogeochemistry—Past and Present, vol. 379 (eds. J. P.Amend, K. J. Edwards and T. W. Lyons). Geol. Soc. Am.,Geol. Soc. Am. Spec. Publ., pp. 1–16.Brunner B. and Bernasconi S. M. (2005) A revised <strong>isotope</strong>fractionation model for dissimilatory <strong>sulfate</strong> reduction <strong>in</strong> <strong>sulfate</strong>reduc<strong>in</strong>g bacteria. Geochim. Cosmochim. Acta 69(20),4759–4771.Brunner B., Bernasconi S. M., Kleikemper J. and Schroth M.(2005) A model for oxygen and sulfur <strong>isotope</strong> fractionation <strong>in</strong><strong>sulfate</strong> dur<strong>in</strong>g bacterial <strong>sulfate</strong> reduction processes. Geochim.Cosmochim. Acta 69(20), 4773–4785.Canfield D. E. (1989) Reactive iron <strong>in</strong> mar<strong>in</strong>e sediments. Geochim.Cosmochim. Acta 53, 619–632.Chernyavsky B. and Wortmann U. G. (2007) REMAP: a reactiontransport model for <strong>isotope</strong> ratio calculations <strong>in</strong> porous media.G 3 8(2), Q02009. doi:10.1029/2006GC00144.Chiba H. and Sakai H. (1985) <strong>Oxygen</strong> <strong>isotope</strong> exchange betweendissolved <strong>sulfate</strong> and <strong>water</strong> at hydro<strong>the</strong>rmal temperatures.Geochim. Cosmochim. Acta 49, 993–1000.Coleman A. S., Blake R. E., Karl D. M., Fogel M. L. and TurekianK. K. (2005) Mar<strong>in</strong>e phosphate oxygen <strong>isotope</strong>s and organicmatter rem<strong>in</strong>eralization <strong>in</strong> <strong>the</strong> oceans. Proc. Natl. Acad. Sci.USA 102(37), 13023–13028.Cypionka H. (1989) Characterization <strong>of</strong> <strong>sulfate</strong> transport <strong>in</strong>desulfovibrio desulfuricans. Arch. Microbiol. 152, 237–243.Dellwig O., Böttcher M. E., Lip<strong>in</strong>ski M. and Brumsack H. (2002)Trace metals <strong>in</strong> Holocene coastal peats and <strong>the</strong>ir relation topyrite formation (NW Germany). Chem. Geol. 182, 423–442.Feary, D. A., H<strong>in</strong>e, A. C. and Malone, M. J. (2000) GreatAustralian Bight: Cenozoic cool-<strong>water</strong> carbonates. In Proceed<strong>in</strong>gs<strong>of</strong> <strong>the</strong> Ocean Drill<strong>in</strong>g Program, Initial Reports, 182.Feary, D. A., H<strong>in</strong>e, A. C. and Malone, M. J., et al. (2000b). InProceed<strong>in</strong>gs <strong>of</strong> <strong>the</strong> Ocean Drill<strong>in</strong>g Program, Initial Reports Leg